Anterior Cruciate Ligament Injuries
An Anterior Cruciate Ligament (ACL) tear is one of the most common and serious sports-related injuries1 with over 200,000 ACL injuries diagnosed in the United States each year. 2 3
The rate of injury has been increasing ~ 1.3% annually4 with rates nearly doubling over the last decade! 5 6 7 8
Researchers in Victoria Australia monitored ACL injuries in youth athletes over the course of a decade (2005-2015) and reported a 148% increase!
The author of this study suggested that the “greater demands being placed on youth athletes through increased training, younger sports specialization and emphasis on year-round competitive play have led to an increase in the diagnosis of sports-specific knee injuries”. She goes on further to emphasize, “it is clear that the prevention of ACL injuries is of increasing importance”… a point that we will return to further along in this post – but it is worth repeating!
There is no doubt that sports participation is a strong risk factor for ACL injuries. Actions such as jumping, cutting & pivoting put the athlete at a particularly higher risk. 9 10 ACL injury rates are highest in soccer, football, basketball, skiing and gymnastics.
Additionally, female athletes are at an elevated risk of injury ranging from 2.4 to 9.7 times more likely than their male counterparts depending on which study is referenced. 11 12 13 14 15 16 The risk of re-injury after ACLR is elevated for female athletes.Up to 4x as high for the ipsilateral (same side) knee, and 6x as high for the contralateral (opposite) knee within the first year of participation after surgery when compared to male athletes.17 18
Why are female athletes at greater risk?
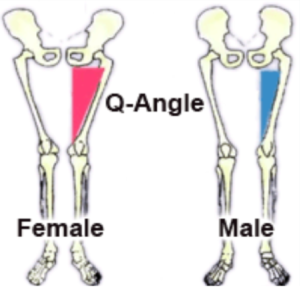
The research into why female athletes are at a greater risk points towards multiple factors:
– Structural/Anatomical differences in the knee (such as a narrower ‘notch’ [or groove] that the ACL courses through)19
– Boney/Structural differences affecting lower limb alignment (such as a wider pelvis leading to altered stresses at the knee and/or weaker hip muscles)20 21
– Ligamentous Laxity22
– Altered muscle balance across the knee as a result of Hamstring and Gluteal muscle weakness.
– Altered Neuromuscular Control – Including muscle firing imbalances and movement patterns unique to the female athlete that place higher stresses at the knee…23 24
*** Note these last two ‘risk factors’ are important as they are the only factors that are ‘modifiable’. We believe this is of the utmost importance as studies have shown that re-training these movement patterns can lower the incidence of female ACL rupture to equal that of males.25
Anatomy of the ACL
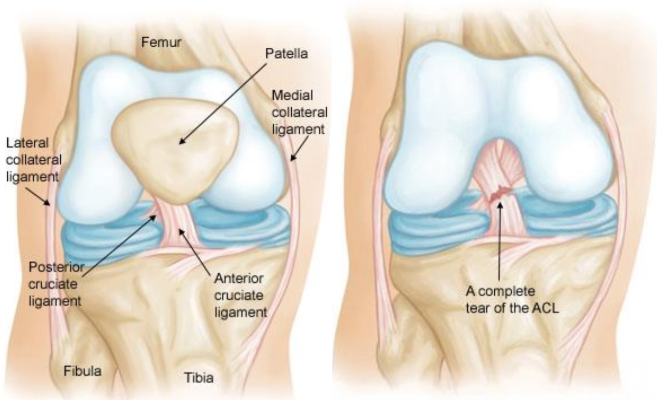
The ACL is a band of dense packed fibers inside the knee joint that connect the distal (bottom) end of the Femur (the thigh bone) to the proximal (top) of the Tibia (shin bone). The ligament courses from a ‘notch’ (or fossa) on the femur, running anteriorly (forward), medially, and distally down to the tibia.26
The tibial attachment of the ACL is somewhat wider and stronger than the femoral attachment27 with an insertion footprint ~ 20% larger than that of the femoral attachment.28
The ACL is often considered as having two portions or ‘bundles’.29 (Some have proposed a three bundle “model”, however the two bundle “model” is generally accepted as the best representation to understand ACL function). These divisions are referred to as the anteromedial (AMB) and posterolateral (PLB) bundles.
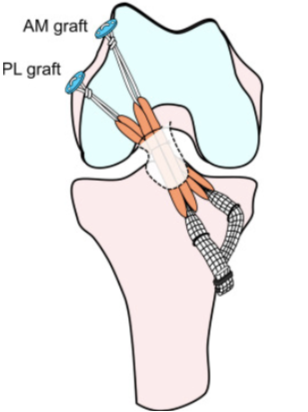
The anatomy of these bundles differs slightly. Also, as the ligament courses distally to the tibia, it turns on itself, creating a slight outward (lateral) spiral configuration. This, in combination with the multiple attachments across the insertion ‘footprint’, allows the ACL to provide stability in many directions and throughout a wide range of knee range of motion (ROM)…30
This anatomy is important when we consider the ability of a reconstructed graft to mimic (or not) the function of the natural ACL.
The ACL primarily consists of 90% Type I collagen (very strong and resilient fibers).31
The ACL also contains many sensory nerve endings, signifying its importance for proprioception (balance) and kinesthesia (position sense).).32 33 Receptors in the ACL also affect local muscle function around the knee in a process that has been described as the “ACL reflex.”34 Loss of this feedback from an ACL rupture results in altered motor function and local muscle weakness (particularly in the Quadriceps muscle)35 – functions that must be returned through deliberate rehabilitation! (Whether reconstructed or not).
The anatomy of the ACL is actually much more nuanced (and impressive!) than the above summary. For more on ACL anatomy (if you’re really looking to geek out on the details) click here.
Function of the ACL
The ACL is a key stabilizing structure in the knee joint.
As previously mentioned, the two ‘bundles’ differ in functionality slightly, allowing the ligament to provide stability in multiple positions/directions. Specifically, the PLB is most taught near full knee extension (while the AMB is somewhat “lax” in this position). As the knee flexes, the AMB becomes progressively more taught, leaving the AMB as the primary restraint to anterior tibial translation (the mechanism that commonly injures the ACL).36
See video for a better understanding of the ACL Function37
The ACL is able to provide resistance in various angles of knee motion, but primarily resists:
A) Anterior (forward) translation of the tibia on the femur (providing ~ 85% of the stabilization against this “anterior drawer” force); 38
AND
B) Rotational Forces about the knee. 39
Even more explanation here:40
How Do These Injuries Occur?
Only ~ 30% of all ACL injuries are the result of direct contact (with another player or object). The remaining 70% result from non-contact injuries (e.g. planting, pivoting, landing).41 42
A direct hit to the knee can result in an ACL tear, however, the more common injury results when the athlete:
- Twists his/her knee while keeping the foot planted on the ground.
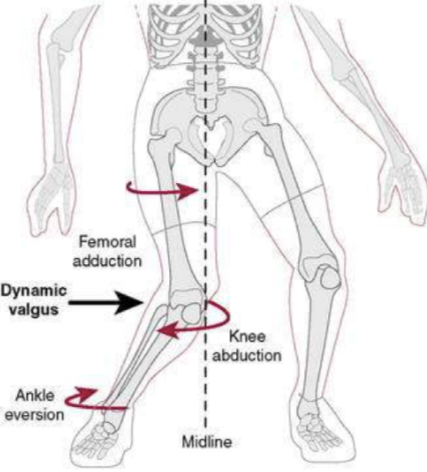
- Lands or cuts suddenly, with the knee in poor alignment (see ‘Dynamic Valgus’ explanation in video above).
- Stops suddenly while running.
- Jumps and lands with the knee extended.
Specifically, there are three common factors, very often associated with ACL rupture: 43 44 45 46
 A strong contraction of the quadriceps muscle over a slightly flexed knee
A strong contraction of the quadriceps muscle over a slightly flexed knee- A marked “Valgus Collapse” (knocking in) of the knee
- Excessive internal rotation (rolling in) of the Femur
Often, the problematic movement known as “Dynamic Valgus” can result from the athlete being off balance, bumped by an opponent, or actively avoiding contact with an opponent. These unlucky circumstances may be compounded by 1) inadequate muscle strength to pull one’s self out of this position; and/or 2) poor neuromuscular coordination or reaction time.47
Other mechanisms, such as hyperextension, or ‘Sports Specific’ mechanisms, such as the “Phantom Foot” phenomenon in skiers, also exist.
ACL Injury is typically classified as a grade I, II, or III sprain48
Grade I Sprain:
– The fibers of the ligament are stretched, but there is no tear.
– There may be little tenderness and swelling.
– The knee does not feel unstable or give out during activity.
– There is no laxity on clinical examination.
Grade II Sprain:
– The fibers of the ligament are partially torn.
– There may be some tenderness and/or moderate swelling.
– The joint may feel unstable or give out during activity.
– There may be some laxity on clinical examination.
Grade III Sprain:
– The fibers of the ligament are completely torn (ruptured); the ligament itself is torn completely into two parts. (An ACL “avulsion” occurs when the ACL is torn away from the bone. This type of injury is more common in younger children.)49
– There is tenderness/pain, however the pain may not be severe (especially when compared to the seriousness of the injury).
– There may be a little swelling or a lot of swelling (typically the onset of swelling is quick – within 1-2 hours of the injury).
– The ligament cannot control knee movements. The knee typically feels unstable and may give out at certain times.
– There is laxity on clinical examination.
How Does It Feel?
While the severity of pain can vary greatly, typically the athlete will report extreme pain.
You may also hear a loud “pop” or snap. Most athletes report this as an unmistakable sign that ‘something tore’.
You may not be able to walk due to inability to support your weight on the injured side – although this too is not present 100% of the time. Stories of athletes attempting to “walk it off”, continue skiing, or even try to return to the playing field are not unheard of. Other times, the knee will feel completely unstable and give way if you attempt to put weight through it.
Usually, the knee will swell immediately (often within a matter of minutes, but always within 1-2 hours on the long end).
How is it Diagnosed?
A Physical Therapist, Athletic Trainer or Orthopedist can perform tests on the knee to test for the presence of the ACL. These tests are much more accurate when performed immediately (before swelling sets in). Most commonly a “Lachmans Test” will be performed with other additional tests if indicated.
If an ACL tear is suspected, an MRI will often be ordered to confirm. In addition to identifying the integrity of the ligament, the MRI can aid in detecting the presence of meniscus tears, articular cartilage damage and bone contusions (a “bone bruise”). These associated injuries can affect recovery rate and ultimate recovery potential.
Can you Prevent them?
Injury prevention interventions have been shown to be highly effective at reducing ACL injury rates. 50 51 52
The most well-known (and well-studied) programs include the Prevent Injury and Enhance Performance (PEP) program, the Fifa1 and Fifa 11+ programs, and the Knee Ligament Injury Prevention program. These programs seek to improve an athletes conditioning, balance, strength and movement patterns. There are similarities among these programs and specific component pieces that are likely more important than others. Of great importance is the correction of faulty movement patterns.53
“Intervention programs aimed to reduce the risk of ACL injury are based on training safer neuromuscular patterns in simple maneuvers such as cutting and jump landing activities.” 54
However, you can’t simply jump into any old program and go through the paces.
Before entering into a generic program, it is so important for the athletes to be screened for movement patterns associated with injury. This is to ensure that detrimental movement patterns are not used (and thus not getting reinforced) during those programs. Such ‘screenings’ have been shown to help identify athletes at higher risk of future ACL injury.55
After receiving the diagnosis of an MRI tear, the radiology report may make mention of one such ‘associated’ injury that is quite frequently (84% – 98%) found with an ACL tear – a bone bruise, or contusion.56 57 58
This subcortical bruising can be observed on MRI and is thought to represent tiny ‘micro’ fractures of the spongy bone towards the end of the femur or tibia.59
These appear to be especially severe when the injury involves structures in addition to the ACL (MCL and Meniscus) 60
There is some disagreement in the literature regarding the impact of these contusions. Some suggest that they are unlikely to contribute significantly to pain levels or ultimate functional outcomes.61 Other studies have suggested higher pain levels associated with more extensive bruising.62
While the majority of bone bruises resolve on their own, long term changes in tissue health can occur. There is also some disparity in the literature regarding the expected time frame for these to heal. Overall, we can say that they heal slow and studies have reported persistence on MRI for ‘years’.63 and the relationship to Osteoarthritis development remains unclear.64
Impact of Boney Geometry
A steep ‘slope’ of the tibia (shin bone) has been shown to put an athlete at an increased risk of ACL tear.65 66 67 68
This has also been shown to increase the risk of re-injury after first time tear and reconstruction;69 70 71 and contribute to “less favorable” long term outcomes after reconstruction.72
One very recent study73 of athletes who underwent ACLR found that those with a posterior tibial slope (angle of the shin bone) > 12 degrees increased their likelihood of re-tear 11-fold compared to the athletes with a slope < 12 degrees. That’s a huge risk factor! [note]Salmon, L. J., Heath, E., Akrawi, H., Roe, J. P., Linklater, J., & Pinczewski, L. A. (2018). 20-Year outcomes of anterior cruciate ligament reconstruction with hamstring tendon autograft: the catastrophic effect of age and posterior tibial slope. The American journal of sports medicine, 46(3), 531-543.[/note]
Those with a steep slope demonstrated a ‘graft survival’ of 22%… not good odds at all! This is a relatively new focus of research, and these factors will likely become a focus point in the near future when considering Return to Sport recommendations, and/or surgical considerations.
* As a side note, one of the world’s foremost expert and leading researcher on this topic is UVMMC’s very own Dr. James Slauterbeck.
Risk Factors for ACL Injury
The key to understanding ACL injury prevention, is to understand the risk factors that increase the likelihood of this injury.
Risk factors for ACL injuries include:
- Environmental – This includes the type of sport and the environment in which the sport is played, including factors such as shoe wear and/or playing surface type. The concern with respect to the shoe/surface interface is anything that creates an increase in ‘torsional’ (rotational) traction . Pooled data from three separate studies suggest that the athlete’s chances of injury are approximately 2.5 times higher when higher levels of rotational traction at the shoe-surface interface are present.74 Rotational traction can be affected by everything from the amount of torsional resistance in artificial turf,75 to the amount of rainfall and evaporation rate76 to the number of cleats on a boot.77 Essentially if the foot gets ‘stuck’ on the floor when pivoting (rotating), this is a problem.
- Non-Modifiable Anatomical Factors – Essentially, we are looking at skeletal morphology and alignment here. The local boney structure of the knee can predispose the athlete to injury (e.g. a narrow femoral notch, or a steep tibial slope).
- Modifiable Anatomical Factors – This is where the money is! Balance in strength between the Hamstring muscles and the Quadriceps muscles, proximal (hip/core) strength, motor control & coordination during dynamic tasks.
Let’s look at these factors in a bit more detail…
Balance in leg muscle strength (between the thigh muscles [Quadriceps] and the muscles along the back of the leg [Gluts and Hamstrings]), movement coordination and raw conditioning are all related to ACL injury and opportunities for sports medicine clinicians to intervene and reduce injury risk. 78 79 80
Poor ‘proximal’ strength (core, hips, etc) is also a risk factor for ACL injury.81 82 83 84
Poor motor control over the lower limb with movement such as landing, cutting and deceleration are particularly important and are readily observable with proper training and an appropriate screening method. Impaired coordination of the lower limb in the frontal (side-side) plane and transverse (rotational) plane lead to an increase in ”Dynamic Valgus” during these movements. Research has shown this be associated with ACL injury in both the ACLR and the healthy population.85 86 87 88 89 Researchers have also shown that poor landing control can even predict ACL injuries (specificity 73%, sensitivity 78%) when excessive ‘Dynamic Valgus’ is found. 90
Surgery or No
If the ACL is torn – you need surgery, right? Well, maybe yes, maybe no.
The decision whether a reconstruction is best for you or not, is a complex one. Below we try to offer some information to inform you about the pro’s and con’s of each approach, and highlight under which specific individual circumstances one approach may be better than another.
Because the ACL does not heal when torn, surgical reconstruction is often suggested as the “standard” treatment in the field of sports medicine – particularly in the United States.91 Surgery is often recommended, in particular, for the adolescent athlete92 which, given the increased participation in pivoting sports, and the potential protective value of a reconstruction against secondary damage93 is widely accepted as an appropriate recommendation for this group. However, some consideration should also be given to the considerable amount of evidence that re-tearing of grafts is significantly more common in youth athletes.94 95 96 97 98 99
While a reconstruction is widely recommended, and offers many benefits – both known and assumed – surgical reconstruction may not be necessary for everyone. Some patients seem to ‘cope’ very well without an ACL. There are many reasons theorized for this, but the short story is, a subset of patients recovering from a ruptured ACL seem to recover their ‘functional stability’ quite well (e.g. the knee does not ‘give out’). Sports medicine experts used to talk about the “rule of thirds” – one third of individuals can resume previous recreational activities without reconstruction (“copers”), one third can manage without reconstruction by modifying/lowering their activity level (“adapters”), and one third require reconstruction because of recurrent giving-way episodes in activities of daily living (“noncopers”). 100 This declaration was based mostly on clinical experience and observation. However, in the past decade or more, considerable research has contributed to a deeper understanding.
What is clear, is that the majority of individuals with an ACL-deficient knee lack dynamic knee stability and will be unable to return to all activities without surgical intervention. But! Some individuals seem to have the ability to dynamically stabilize their knee, without an intact ACL, even during pivoting sports activities.101 102 103 104 These people can be defined as ‘copers’ and they may be able to resume all preinjury activities, including sports, without episodes of knee giving-way and without surgery.105 106
Others seem to be able to return to most activities shy of cutting, pivoting sports and can be successful only modifying their activity levels. These patients can be defined as ‘Adapters’. This groups seems to represent the vast majority of those who are managed non-operatively, successfully avoiding re-current instability through a combination of structured rehab and lifestyle modifications.107 108
There are screening procedures that can be utilized to attempt to predict an athlete’s ability to ‘cope’ early on, however, we often cannot know for sure without a trial period of conservative management.
But, not everyone ‘copes’ well without an ACL. If the knee continues to feel unstable, this is more likely to be the case. Even for those motivated to avoid surgery, after a bout of Physical Therapy (up to 3 months) to reduce inflammation, return mobility, make sure the muscles surrounding the knee ‘wake back up’ and attempting to regain neuromuscular control of the knee, patients may find that they are unable to function successfully, at the desired level, without a reconstruction. Many factors that determine your ability to ‘cope’ may be completely out of your control (such as the geometry of your knee structure).109 110 Additionally, injuries to the ACL rarely occur in isolation. Often the presence and extent of additional injured tissues (e.g. articular cartilage, meniscus, etc) may weigh heavily on the surgical decision.111
On the flip side, there is a sub-group of injured athletes who may not be good candidates for reconstructive surgery (even if they want it!). This may include athletes over the age of 40 (especially those facing little exposure to high demand activities), patients with advanced osteoarthritis, and those unwilling to comply with post-operative rehabilitation protocols.
Research on Identifying “Copers”
One study performed in Sweden tracked 200 individuals with unreconstructed ACL tears over a 15-year period. With early activity modification and neuromuscular rehabilitation, a ‘satisfactory’ activity level was achieved in the majority of individuals, limiting the need for surgical reconstruction to only 23%.112
One study performed at the University of Delaware categorized individuals as ‘potential copers’ or ‘non-copers’ early (within 6 weeks) after injury. Those sub-classified as ‘non-copers’ based on early screening > 90% went on to have surgery. While those classified as “potential” copers demonstrated a 43% chance of avoiding surgery.113
Another study from Norway looked at 125 highly active individuals with ACL tears, and sub-classified their participants (based on a very similar ‘screening’) as copers and non-copers. The researchers reported that 70% of those classified as likely non-copers ended up reporting “excellent” results without surgery! The authors question the wisdom of an early exclusion from potential non-operative management.
| Higher Likelihood of ‘Coping’ (with an ACL deficient knee) | Lower Likelihood of ‘Coping’ |
|---|---|
| Older / Lower Demands | Younger/ Higher Demands |
| Activities do not include cutting, pivoting, or rapid deceleration | Sports participation includes cutting, pivoting, and/or rabid deceleration |
| Minimal episodes of recurrent instability after the initial event | Multiple episodes of recurrent instability after the initial event |
| ‘Healthy’ movement pattern early after injury | Poor muscle control and movement patterns after injury |
| Isolated ACL tear | ACL tear with (+) damage to secondary tissues |
| Willing to attempt a trial of Physical Therapy | Unwilling to delay surgery if there’s a chance surgery will be necessary |
The decision to have surgery or not is a complex one and should be done in consultation with experts. Due to the increased risk of ‘secondary damage’ to the ACL-deficient knee, the American Academy of Orthopedic Surgeons (AAOS) recommends that, if a surgical reconstruction is indicated, it should be performed within 5 months of the initial injury.123
Secondary damage to the articular cartilage and/or meniscus carries the risk of serious, adverse long-term effects that may significantly impact the individual’s quality of life. Delayed ACL reconstruction has been shown, in multiple studies, to be associated with an increased frequency of these injuries.124 125 126 127 128 129 130
It is important that all risks are considered so that injured athletes can make an informed decision.
Similarly it is important that an individual’s understanding of the benefit to surgery be evidence-based, and an athlete’s expectations be realistic.
Expectations
There was a time, not so long ago, when a ruptured ACL was considered a career ending injury. With advancements in both surgical techniques and rehabilitation approaches, we have come a LONG way.
Nowadays the pendulum may have swung too far in the opposite direction – athletes often assume a full recovery is a given. The reality, however, is a different picture altogether.
High Profile Professional Athletes
We see highly visible athletes like Adrian Peterson (when he was playing for the Minnesota Vikings) return to the NFL 7 months post-op and perform at an elite level and we think – “that’s what an ACL reconstruction recovery curve must be now”. Sadly this is not the reality for most athletes. And, for many, it should not even be the goal.
Consider:
a) Adrian Peterson (AP) had a “clean” ACL injury (no cartilage or meniscus damage)
b) AP had a world class team of surgeons, physical therapists, trainers, etc
c) AP has resources we don’t – Daily massages, a nutritionist preparing meals to reduce inflammation and promote recovery, etc.
(How many of the readers out there sleep in a hyperbaric chamber at night?)
d) It is his JOB to recover. He can dedicate 4-6 hours a day to recovery & rehab!
e) And, oh yeah, he’s a world class athlete.
Popular media rarely highlights the athletes who do not recover!
Pre-game specials never take you into the home of the NFL player who’s ACL injury proved to be a career ender (or at least a career changer) – Terrell Davis, Daunte Culpepper, Jamal Anderson (not to mention a host of NBA players!)
Some reality check stats
More recently, sports medicine specialists have begun to recognize that the majority of individuals who undergo an ACLR do not return to their pre-injury levels of sport, and many do not even try to return.133 134 Some studies report that up to 2/3 of athletes who undergo ACL reconstruction do not return to their preinjury levels of sports participation.135 136 A meta-analysis of 7556 subjects performed in 2014 demonstrated a 55% return to competitive sports.137 Not very encouraging statistics!
Additionally, for those who do attempt to return, many athletes require more than 12 months to successfully return to sport.138 In fact, on average, only ~ 1/3 of amateur athletes return to sport (at pre-injury levels) within 6-12 months.139 140 141 This number rises to ~ 2/3 when the timeline is expanded to 2-7 years142 highlighting the fact that a large proportion (~50%) of athletes who do successfully return to sports, require an extended recovery period. Further, the return to sport rates “drop markedly” if you are unlucky enough to suffer a second ACL injury.”143 144
Contrast this to research suggesting that, pre-operatively, 90% of athletes undergoing an ACLR estimate that their rehab and return to sport will take less than 12 months.145 The authors of one study146 state plainly that “Clinicians and patients may need to reassess their expectations for rehabilitation and return to the preinjury level sport after ACL reconstruction”, given that all of the athletes in their study “intended to return to sport and received surgical clearance to return to sport from 9 months after surgery”, however, 69% had not returned 1 year after surgery.
(It is also important to remember that the type and level of sport played plays a major role in influencing return to sport rates – for example, it is likely much more challenging to return to soccer at a high level then it is to return to competitive cycling.)
Bottom line, the likelihood of a successful return to sport are less favorable than many “expect”.
On a more optimistic note…
With all of this said – the “average” recovery is not necessarily what you should expect. If you are willing to put in more work than the average person then your results may not be “average”.
Overall, outcomes (including successful return to sport) are improved in younger147 and higher level athletes.148 149 150
In fact, a recent systematic review found that among ‘Elite’ level athletes, the Return to Sport (RTS) rate is over 80%151 – much more favorable than the wide range of RTS rates among mere mortals!
Success rates, for a full return to sport, have been shown to be increased among athletes who show increased motivation and a strong desire to return to sports.152 153
With respect to recovery, rehabilitation and a return to prior level of activity among athletes, “the amount of time and effort (an athlete is)… willing to invest to return to sport” plays a significant role in determining success.154
If you have experienced an ACL injury, the sports rehabilitation specialists at VASTA can provide expert-level guidance to optimize your recovery.
Return to Sport rates by Sport (for Professionals)155
Return to Sport rates for individual sports (among “Elite” athletes) are ~ 85% for Soccer players; ~ 78% for American Football players; ~ 82% for Basketball players.
Return to Sport time frame for individual sports (among “Elite” athletes) were reported as 6 – 10.2 months for soccer players; 8.2 – 13 months for American football players; 10.7 – 11.8 months for basketball players; 7.8 – 9.8 months for ice hockey players.
* It is important to remember that these results apply to professional athletes. The superior skill,156 physical fitness,157 balance/proprioception,158 159 unique psychological profile,160 ready access to the highest quality healthcare,161 and greater financial incentives to return to sport make it impossible to apply these results when setting expectations to the recreational athlete. However, it does highlight the finding that with proper care, guidance and motivation, our results can be elevated.162
Surgery
If an injured athlete is determined to require reconstructive surgery, a few decisions need to be made with regard to surgery timing, and the need for ‘prehabilitation’ will factor in. It is recommended that before having surgery, the injured athlete have minimal swelling (effusion), full return of knee extension mobility, and a good return of quadriceps muscle function. Post-surgical recovery are adversely affected if these benchmarks have not been met.
The surgery itself generally consists of 5 steps: 1)graft harvest/preparation; 2) diagnostic arthroscopy; 3) drill tibia (shin bone) tunnel (for graft attachment); 4) drill femoral (thigh bone) tunnel (for graft attachment); and 5) fixation of graft at each end. The purpose of the diagnostic arthroscopy is to identify any additional injuries in the knee (e.g. cartilage damage, meniscal tears, etc.). Here the surgeon can perform any additional procedures necessary based on the arthroscopy’s findings. The tunnel drilling and graft fixation techniques vary somewhat depending on the surgeons training, preferences and choice of graft material.
The video below demonstrates an ACL Reconstruction (ACLR) using the patient’s own Hamstring tendon as a ‘graft’ to replace the ruptured ACL, but the approach is very similar for other graft options as well.163
Graft Choices
Graft material for ACLR are typically autografts or allografts. Autografts are tissues harvested from the athlete themselves and these include hamstring tendon grafts, quad tendon grafts, and patellar tendon grafts (often referred to as a bone – patellar tendon – bone (BPTB) grafts because each end of the harvested tissue includes a bone ‘plug’ taken from the patella and tibia (shin bone). Allografts include tissues from outside sources (typically a cadaver) and may be harvested from muscles/tendons of the shin, achilles tendon, or BPTP.
Each option brings with it it’s unique advantages and drawbacks.
BPTB Grafts. One major advantage of BPTB grafts include the strongest early fixation due to bone healing (the bone ‘plug’ heals to the boney tunnel quickly). Also, the BPTB graft does not compromise the hamstring muscles (an important protector of the ACL). The mechanical properties of the BPTB graft is strong, with ‘ultimate failure strengths’ and stiffness similar to the native ACL.164 or better (up to 168% as strong).165 Due to the strength and strong fixation, the BPTB graft is commonly referred to as the “gold standard” in ACL reconstruction.
The disadvantages may include direct anterior knee pain and delayed early recovery. Additionally, matching the length of the graft to that of the tunnels can be problematic. Some of these disadvantages can be countered by use of a contralateral (opposite side) patellar tendon graft, however surgeons are often reluctant to utilize that approach as it leaves the patient with ‘2 bad knees’.166 In addition to ‘donor site’ pain, BPTB grafts present with an increased (albeit small) risk of patellar fracture.167 168
Zelic et al & Abbas et al 169 170 found that there were no significant differences between BPTB and HS tendon grafts with regards to knee stability. Individuals who used a BPTB graft had a statistically greater chance of developing anterior knee pain at 6 months, but this did not seem to affect the patients overall outcomes.
Hamstring (HS) Grafts. One major advantage to a HS graft includes quicker post-op Quadriceps control. Also, these patients tend to have less anterior (front-side) knee pain (compared to BPTB). This allows for easier early rehabilitation.
Also, the graft itself is stronger. The hamstring graft can utilize multiple strands, increasing the strength of the graft to greater than all other graft types (at time of implantation).171 Unfortunately, this advantage is somewhat insignificant due to the hamstring grafts (having no ‘bone plug’ at its ends) having their weakest point at the attachment site.
The disadvantages include decreased hamstring activity (hamstrings are important restraint to anterior translation, and thus a protector of the ACL), decreased hamstring strength (especially in positions of deep flexion), inconsistent graft quality, and the need for suspensory fixation (no bone-to-bone fixation).
Allografts. Allografts have many advantages. The surgery is faster (no need to harvest a graft from patient). The early rehabilitation is accelerated. Also, (if it’s important to you) they are more cosmetic (smaller scars).
The disadvantages are that they are more expensive, they place the patient at higher risk for infection, and they don’t “incorporate” as well or quickly as autografts.
Artificial ligaments grafts (such as the Ligament Advanced Reinforcement System – LARS) have shown favorable clinical outcomes without donor site morbidity. More research remains to be done, but we may see increased use of artificial grafts in the future.
“Single Bundle” Vs “Double Bundle”
As the understanding of the anatomy and biomechanics of the ACL has improved, so has our understanding of surgical reconstruction techniques.
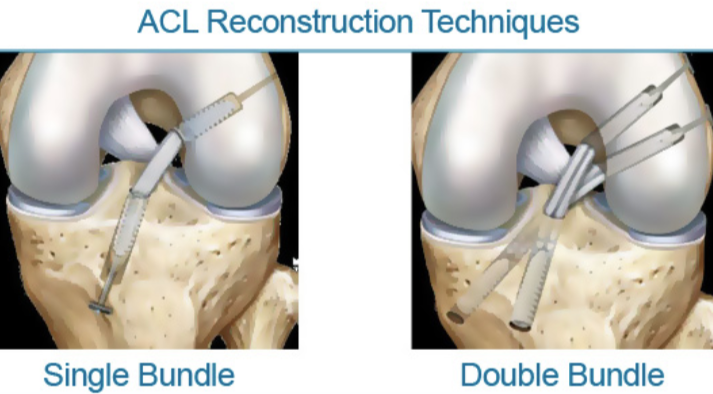
A so-called single-bundle technique has long been referred to as the “gold standard” in ACL reconstruction techniques.172
A single-bundle graft has been shown to have good anterior stability but lacks any contribution to rotary and frontal (side-to-side) stability. A native ACL includes 2 bundles ( some suggest 3) – an anteromedial and posterolateral bundle – and this unique anatomy allows it to prevent anterior translation (as discussed above) and provide some contribution to rotatory and frontal plane stability. More recently, there has been a push to increase the utilization of the double-bundle technique. With the addition of another bundle in the graft, the surgically reconstructed knee can have increased stability in multiple planes as it more closely mimics the dual bundle form of a natural ACL.173 174 One of the biggest drives behind this push, is the hope that these new “double-bundled” techniques may help reduce very small shearing forces within the knee post-ACLR and thus decrease the onset and progression of knee osteoarthritis (OA) in patients post ACLR.
Post Traumatic Osteoarthritis
The research suggests that an individual’s likelihood of developing knee osteoarthritis (OA) after ACLR is elevated. Generally speaking, > 20% of individuals 10-years out from surgery will develop radiologically observable OA, and > 50% will develop OA 20-years out.175
What about really young kids?
“The management of pediatric ACL ruptures is somewhat debatable.”176 The primary concern being the risk of provoking growth deformities by performing surgery in the presence of ‘open’ growth plates. Such ‘transphyseal’ surgeries are sometimes performed, but the research is inconclusive regarding the risk : reward benefit.
Sometimes surgery will be delayed until skeletal maturity; however, the laxity associated with an ACL-deficient knee places the child athletes increased risk of meniscal and cartilage damage177 (as discussed in the adult population above).
If a reconstruction is considered, Frank & Gambacorta found improved clinical outcomes with early surgical reconstruction versus delayed for the pediatric population. They suggest that “the potential for growth abnormalities can be decreased by use of soft tissue grafts (not BPTB) in transphyseal tunnels.” 178
Graft Healing
“When making the return to sports decision, a primary factor to consider is prevention of recurrent injury by ensuring that the graft has healed appropriately and is capable of withstanding the demands placed upon it.”179 After implantation during an ACL “Reconstruction” procedure, the graft begins to undergo a process known as “ligamentization”. This refers to the morphological changes that take place as the graft tissue transitions from its natural state to a structure that more closely approximates (but does not ever fully mimic exactly) the structure of the native ACL. This ligamentization enables the graft to better withstand the stresses placed on it (stresses that the native ligament is uniquely qualified to withstand).180 181 182
There are 3 phases in graft ligamentization: Incorporation, Revascularization, and Graft Healing/Maturation. The time frame is somewhat general, and highly variable 183 184 but incorporation typically occurs in the first 3 weeks.185
Initially, the graft is actually stronger than a normal ACL and it is the attachments to the bone (in the tunnels) that are of concern.186
Immediately the graft begins to degenerate and the cells (fibroblasts) die. The tissue left behind is referred to as a “scaffold”. Thus, the graft’s primary purpose may be thought of as merely a scaffold for new cell proliferation to occur.187
During this phase the graft is avascular (void of blood flow), but very quickly becomes enveloped by native tissue and vascularized. (In fact, the graft eventually becomes hyper-vascularized until the ligamentization process is complete, at which point the vascularity returns to normal levels.188
Revascularization occurs from week 3 to about week 16 as the capillaries invade the graft from the the surrounding tissue (the synovium). The patient’s own cells (fibroblasts) migrate into the graft tissue.
Depending on the research cited, the graft may be at its weakest point between 2-6 weeks189 or as late as weeks 8-10, during the revascularization phase.190 So, it is important to avoid over-stressing the graft during this time frame.
Graft Healing/Maturation begins after revascularization. The graft strength drops to as low as 11% of a normal ACL, but as the graft matures there is a gradual build-up of collagen content strengthening the graft. The collagen fibers begin to re-align themselves to orient themselves in the direction that best enables them to resist forces placed on the ACL.
The ‘full maturation’ is the timing at which the graft finally approximates the strength and resiliency of a native ACL. This stage has been shown to begin sometime between 9 and 18 months.191 192 Some studies have shown this time frame to be earlier in autografts,193 but other studies suggest that the graft will not reach normal ACL tensile strength until at least over a year (if at all) according to some sources.194
There is some indication that advanced MRI images may be useful in predicting graft maturity – but the research supporting this practice has been limited largely to animal models.195 196 With improvements in MRI technology, this may assist in the return to sport decision in the future, however, currently the added value of MRI imaging when making return to sport decisions is equivocal.
Thus, ‘time from surgery’ and graft maturation, in isolation, cannot be used as the sole determinant for judging return to sport readiness.197
Further, and most importantly, we should assume that any athlete returning to sport prior to 9 months, may be assuming the additional risk of stepping on the playing field with an immature graft.
Post-Op Rehabilitation
Before we begin post-op rehabilitation, you will likely require a period of “prehabilitation” in the time after injury but before surgery. There are several reasons why surgeons often elect to delay ACL reconstruction for several weeks following initial injury.
Adams et al found that patients with full knee extension ROM, absent or minimal joint effusion, no quadriceps lag with a straight leg raise, and a quadriceps index >90% had significantly better post-surgical outcomes.198
The swelling in the knee, at the time of surgery, could impair the healing process following reconstruction and limit post-op ROM.
Multiple studies have demonstrated the importance of regaining full knee extension range of motion and re-establishing a good quadriceps ratio pre-operatively. Decreased extension is a significant complication following surgery that may be linked to further knee damage. By regaining this motion prior to surgery, the patient is more likely to return to higher level activity and decrease the chances of further knee injury. Additionally, it is desirable to regain as much strength as possible before surgery.
“It has been shown that early rehabilitation improves knee function following ACL reconstruction”.199
Once a surgical reconstruction has been performed, there are several milestones we look to reach in rehabbing our patients.
EARLY REHAB PHASE
Initially, we want to decrease knee pain and swelling, restore knee extension ROM, and improve quadriceps recruitment. These early milestones are similar to our prehabilitation goals.
In the initial few days and weeks post-op, the patient may or may not be using a brace (immobilizer). It is not uncommon for a surgeon to recommend only a day or two in the brace. It is also fairly standard practice to use a brace for 2-3 weeks to protect the graft and / or ensure knee extension ROM is maintained. This decision is unique to the specific surgery as well as to the surgeons preference. Overall, when looking at ACL reconstructions, the research suggests little to no benefit to bracing everyone.200 However, it may be of some use in certain cases, especially if terminal extension ROM is difficult to obtain and stiffness is a concern.201
“Following surgical reconstruction, it is essential that the patient achieves full knee extension for maximum functionality. Even a 3-5 degree loss of extension, ROM has shown to negatively impact patient subjective and objective reports and an association with decreased quadriceps strength”202
Athletes who were unlucky to injure their ACL in the 1980’s likely experienced post-operative casting, delayed weight bearing and prolonged limitation of ROM, which lead to significantly increased pain and problematic stiffness (often never fully recovering!)
Luckily, times have changed.
Currently, post-operative training includes an “accelerated approach” – immediate ROM, muscle firing and (often) early weight bearing.
Kruse et al also found that “an accelerated program had no deleterious effects” when post-operative patients could immediately initiate weight-bearing, knee motion 0-90 degrees of flexion, and weight bearing exercises.203
Another reason to achieve full extension and full ROM is to decrease the likelihood of developing osteoarthritis (OA). Decreased knee ROM following ACL reconstruction has been shown to be associated with the development of knee OA.204
It is important to remember that “full ROM” refers to normal ROM for the individual. We can use side-to-side comparisons to determine the achievement of full motion.
Exercises to reduce post-op quadriceps inhibition and promote a return of quadriceps strength is important. While early quadriceps loading has been considered “contraindicated” in the past, there is evidence that they may be safe (even moderately heavy eccentric loading) early on.205
We support the early use of Electrical Stimulation to increase quadriceps recruitment and function very early post-op. Evidence shows electrical stimulation plus exercise improves quadriceps strength greater than exercise alone in this stage.206
We use cutting edge technology such as Blood Flow Restriction (BFR) Training with our patients early in their post-op rehab. The research supporting this modality’s ability to mitigate (or even completely avoid) post-op atrophy in the quadriceps is very promising. We see great results at our facility and encourage anyone interested in more information (including the research supporting BFR’s safety and effectiveness) to check out this link.
A potentially useful neurological component to add to the early rehabilitation stage is cross-exercise. Cross-exercise is a term to describe the phenomena of training the muscles of the uninvolved leg and seeing carryover to the same muscles of the involved leg. In order to reduce the negative effects of immobilization and disuse (muscle atrophy), cross-exercise has been shown to improve quadriceps strength207 and may often be included as an adjunct to other early rehab activities.
Another important goal of early rehabilitation is to have the patient focus on normalizing their gait. Even if crutches are to be used, avoiding a limp is very important as there is a growing consensus that a habitual “quad sparing” (reduced knee flexion while walking) gait is likely related to an increased risk of early OA development. In cases where the ACL injury is isolated (e.g. no meniscus repair or cartilage damage that was targeted during the surgery) we will have our patients weight bearing right away.
Thus, the early stages of rehab are likely to include a focus on reducing effusion, returning extension ROM, attempting to avoid quadriceps atrophy, and returning to a normal gait pattern. Additionally, patients will focus on restoring knee flexion ROM, and initiating weight bearing exercises as well as low-level proprioception (balance) exercises.
Do I Need a CPM Machine?
CPMs are frequently known for their use following total knee arthroplasty, and there has been discussion on their potential for use following ACL reconstruction. Reviews of the literature has found there is no statistically significant advantage to using CPM following ACLR surgery.208 209
However, in certain cases, especially cases where the reconstruction includes cartilage salvage techniques (e.g. meniscus repair, articular cartilage microfracture, etc.) your surgeon may prescribe one.
INTERMEDIATE REHAB PHASE
The intermediate phase of rehab is highly variable. During this phase, the athlete has recovered full motion, has little to no swelling, a functioning quadriceps muscle and a normalized gait pattern. This phase of rehab is highly variable and no time tables will be given here for that reason.
Focused is placed on strength improvements, neuromuscular training of healthy movement patterns that are protective of the knee, perturbation training to build proprioception and reaction time, as well as a graded progression of activities to ‘harden’ the knee to improve patient confidence.
The athlete will be addressing the basic building blocks necessary for athletic activities (a baseline of strength, good motor control with basic movement patterns, etc.) The exercises used through this process will be specially “dosed” out in the right form and intensity. For example, “Open chain” exercises – Exercises performed with the foot off the ground and the leg non-weight bearing – will be used to build Glut, HS and Quad strength. However, heavy firing of the quadriceps in open chain (like a knee extension machine) place a significant load on the graft when performed at shallow knee flexion angles. While your graft is healing and vulnerable, your Therapist will avoid this particular movement and range. “Closed chain” (weight bearing) exercises have many advantages. Loading the leg against resistance in closed chain, places less strain on the graft for many reasons. Your PT will use tricks, such as deeper knee flexion angles and an increase trunk lean210 to further reduce strain on the ACL and allow the athlete to progress activities safely. During the Intermediate phase this will include exercises such as lunges (multi-angles), step-up, squats, Romanian dead lifts, etc.
During this phase the athlete will focus on strengthening areas away from the knee as well – Core, Hips, Foot/Ankle, etc.
Following an ACL rupture the individual experiences decreased knee proprioception (balance / position) sense. During the intermediate phase of rehab, the athlete will spend a lot of time re-developing this joint position sense, balance, and reaction time to perturbations. Exercises that challenge the athletes ‘Dynamic Stability’ will be introduced. The athlete will spend time on unstable surfaces to promote Quadriceps/Hamstring “co-activation”.
Last, the athlete will begin to focus on general conditioning with minimal impact (Bike, Elliptical, Cross-Robics, Pool Running, etc.)
ADVANCED REHAB PHASE
The advanced rehabilitation phase is a natural progression from the “intermediate phase”. Strengthening activities are progressed. Neuromuscular re-education training takes place with more challenging and more dynamic tasks. Balances activities are progressed. Perturbation training becomes more aggressive.
Plyometric drills come into play to build resiliency to impact activities and to ensure proper movement patterns with activities such as jumping, bounding, landing and hopping. Plyometric activities are also implemented to begin to work on explosive strength.
Also, agility drills play a larger role during this phase. Light running, backwards running, light cutting, and varying acceleration / deceleration activities are introduced.
RETURN TO ACTIVITY PHASE
This phase includes the exposure to more “sport-specific drills”. Depending on the specific activities and sports the athlete wished to return to, this phase can take on many forms. For example, a Basketball player may be progressing hop and jump training more than the average player, while an ice hockey player will be spending much more time on a slide board.
During this time, the athlete will be exposed to conditions that closer mimic the real sports environment – catching and throwing balls while performing agility drills, being exposed to unexpected perturbations while performing jump landing drills, etc. Emphasis is placed on the athletes ability to avoid vulnerable positions with intense exertion, and with dynamically challenging tasks (e.g. explosive hopping, sudden changes of direction, etc.).
Also, the speed and intensity of real sports participation is gradually introduced. Leg strength is a primary focus in ACL Rehab, but perhaps just as important, is explosiveness.
“In sport, the ability to generate strength quickly is of utmost relevance to both performance and protection against injury.”211
Several studies have shown that producing a high level of force quickly is more important than just being able to produce a high level of force.212 213
Research has confirmed that a return of max isometric strength (common ~ 6-9 months post-op) is often in the absence of the ability to generate force quickly / explosively, a skill that is uncommon to achieve in 6 months and may take closer to 12 Months (and even then, only when properly trained!).214 This is important as the ‘6-Month’ time-table is a commonly referenced expectation for Return to Sport.215 216
Study of Explosiveness217
Multiple studies have looked at the importance of explosiveness. In one study,218 the researchers studied the rate of force development (RFD) in professional soccer players. Players were followed over a span of 6 seasons. Those who suffered an ACL tear underwent surgery and all started on the same rehabilitation program. However, what was different was that at 6 months post-op the athletes underwent an additional 20 weeks of a training program focusing on RFD.
The authors decided to recommend this additional 20 weeks due to significant deficits in RFD at 6 months. Below are some of the key points of the article:
– One criterion that has been used to determine recovery and readiness to return to sport following an ACL reconstruction is achieving 85% or 90% of the maximal strength of the contralateral limb. However, even when these near-normal strength values are achieved, the rate of successful return to sport remains low.219
– It has been shown that the time required to develop muscular strength in many types of sports activities is considerably shorter (0-200 milliseconds) than that usually required to achieve maximal contraction strength (300 milliseconds or greater).220 221 Researchers have suggested that “adequate muscle activity must occur within a 30-to-70-millisecond window from the onset of joint loading to effectively protect the ACL,222 223 and the failure of this protective mechanism has been suggested to contribute to noncontact ACL injuries.224
So athletes looking to RTS safely need not only to get their max strength back, but their explosiveness too!225 226
Return to Sports (RTS)
The ultimate goal of ACL reconstruction and rehabilitation procedures is “the restoring of the patient’s functional knee stability to prevent re-injury and allow safe return to previous activity levels.227 A major cause of poor outcomes after ACL reconstruction, and our primary concern, is recurrent instability that places the athlete at risk of graft rupture.228
Re-injury rates are high among reconstructed knees as well as to the opposite (previously uninjured) knee229…. Re-injury rates are quite variable depending on which research paper you are reading. Between 3% and 19% of athletes that return to sport re-tear their reconstructed ACL (most estimates are in the upper part of that range), and up to 24% (8% >24%) re-tear their opposite (previously uninjured) knee.230 231 232 233 234 235 Importantly, most of this research was done on ‘isolated ACL tears’, not athletes with co-existing cartilage, meniscus or second ligament tears (which likely leads to even poorer outcomes).
Re-injury rates are considerably higher in youth athletes as compared to their adult counterparts – even in studies when ‘activity level’ is comparable.236 Younger age has been identified as a risk factor for ACL graft rupture of a magnitude of 2 to 7 times, in large multicenter registries from the United States,237 238 and across Scandinavia,239 in places like Sweden,240 241 Denmark,242 and Norway.243
“Reinjury rates are high after pediatric ACL reconstruction.” Authors of one study conclude. A second ACL injury occurred in 1/3 (32%) of the studied group and, importantly, an earlier return to sport was a significant predictor of second ACL injury.244
Said another way – Within the first twelve months after ACLR, the likelihood of suffering a second ACL injury is 15x greater than an average ‘control’ athlete245 and some experts question whether return to play within the first 12 months is advisable at all (regardless of the patient’s progress in rehab).246
One thing is for sure – the re-injury rates are too high!
It is our contention that improper rehab, and improper timing of return to sport (too early) plays a major role in these unacceptably high retear rates! (Especially among youth athletes)
The point in time at which the athlete should return to sport is always a debatable topic. However, a growing contention among researchers suggests that commonly used benchmarks to determine “return to sport readiness” appear to be insufficiently stringent, allowing athletes to return to activities sooner than appropriate – no doubt contributing to high re-injury rates.247 248 249
At VASTA we uses a series of scientifically backed tests to assess the athletes readiness for full speed sports participation on multiple levels.
- The knee must be free of swelling with full, pain-free mobility – including overpressure into end-ranges of motion.250
- Strength Tests – Strength symmetry of at least 90% (compared to uninvolved side) is essential. For ‘High Risk’ athletes – see box to the right – a more stringent number of 95% is used at our facility. Also, we look for a good balance (or ratio) of Quadriceps to HS strength. The HS:Quad ratio should be 85% or better.251 252
- Biomechanical Intactness – a term we used to describe the athlete’s ability to perform a variety of ‘functional tests’ (e.g. shuttle runs, drop-jump, cutting drills, single leg squat, single hop tests, etc.) at a high level (> 90% opposite LE, or pre-injury level if data is available) and with good clean form. It’s not enough to get through the testing. The athlete must demonstrate proper control (‘Dynamic Stability’) of the knee.253
- Psychological Readiness – The athlete must also demonstrate confidence with challenging and explosive tasks.254 Psychological screenings are also used in our facility to determine readiness. (for more on the importance of this – read below)
‘High Risk’ Athletes
Certain characteristics make the risk of graft failure higher than average. These factors include:
- Age > 18
- Revision surgery
- Generalized Laxity (especially in female athletes)
- Participating in high-risk sports (described above)
Return-to-Sport decisions are complex and many factors, unique to each athlete, must be considered. The athlete, and their parents, must be well educated on all the factors going into this decision so that an informed and educated choice can be made that minimizes the risk to the athlete without unnecessarily holding them back.
At VASTA we have created a robust system for our return to sport testing based on:
- The best available research and cutting edge approaches
- Consultation with leading sports clinics across the country
- Collaboration with local sports medicine surgeons
Brace or no?
The use of bracing when returning to sports is a topic of some debate. There is some evidence that a protective effect might be present in certain types of sports / activities. For example, Kocher et al255 studied professional skiers with ACL-deficient knees and found a greater risk of knee injury in those who did not wear a functional brace than in those who did use a brace.
In General, however, the utility is questionable. ‘Functional’ braces have been shown to limit only anterior tibia translation, and only at low loads. The loads placed on the knee is many daily life tasks, to say nothing of cutting, and jumping activities far exceed that which the brace can protect against.
While mechanically, the brace may have little proven benefit, there may be a role for their use if it affects the athlete’s psychological readiness (assuming they are appropriate to be ‘cleared’ for the given task). Braces may improve an athletes balance and position sense (proprioception) by virtue of the compression or skin contact. Some athletes ‘just feel better’ with the brace on – especially the first season back.
It is common for surgeons to prescribe functional braces for athletes following ACL reconstruction. However, due to the conflicting evidence for it’s proven usefulness there is some variability in practice. Your surgeon may recommend a brace for certain activities while your neighbors surgeon does not… do not be alarmed by this!
Psychological Readiness
The role of the psychological component to rehabilitation cannot be overstated. An athlete’s “psychological readiness” is of the utmost importance.256 257 Fear of sustaining a new injury is the number one reason athletes cite for not returning to sport after an ACL injury258 259 260 and individuals who continually express fear of re-injury typically do not return to pre-injury level.261
Among athletes who are successful returning to sports after surgery, those who returned to their preinjury level, had lower fear than those who returned to a lower level sport.262
Studies have shown that ‘psychological readiness’ scores pre-surgery and 4 months post-op predicts pre-injury return to sport status at the one year mark.263
The Importance of Psychological Readiness
One interesting bit of research264 looking into the importance of Psychological readiness, studied youth athletes and provided some very interesting quotes:
Patient #6 – “They told me to do certain exercises. I felt like they were just taking forever to get to the advanced ones, so then I just didn’t want to do the regular ones. And then I felt regression, and I [thought] I need to do more, and they wouldn’t give me more, so . . . I just felt stuck.”
Patient #8 – “It’s not necessarily that . . . I’m terrified of injuring myself again; I also just think how fluky my step was . . . I planted my foot, and I tore my ACL. So, to think about that . . . is a little bit nerve-wracking.”
Patient #1 – “. . . I’m more concerned about the right tearing . . . I’m just worried that I am not going to be as competent as I was and that’s going to reflect in my playing, because I feel if you’re really timid in your playing, then you’re going to get hurt again.”
The study surmised – “…though participants spent an enormous amount of time and effort rehabbing their injury, there was still a major psychological gap to be bridged between post-rehabilitation and return to physical activity”
The rehabilitation of an athlete from an ACLR must instill confidence and achieve the psychological readiness that only comes with a graded progression of sports specific activities and can only be reached when the individual is appropriately challenged and advanced. The Physical Therapist leading the athlete’s rehabilitation must be mindful of this starting from early stages of recovery.
* Side-Note: This same research found that athletes who were well educated about the specifics of their injury, the detail rehab process, what challenges to expect, and what timelines are realistic, are “more likely to maintain a healthy mindset throughout and take a more active role in their rehabilitation”… So, by reading this, you have already started to take steps in the right direction!
“The importance of completing successful criteria-based rehabilitation and returning to sports both at a suitable time and at an optimal level of performance for the adolescent population is imperative.” 265
As you can see, the RTS decision is a complex one, and one that is not ‘time dependent’. It is ‘criteria dependent’. Nonetheless, the injured athletes that walk through our door always want to know ‘when’ they can return to sport. And while, I hope, at this point you can see that this answer cannot be accurately provided early post-op, we can say that, in general, this time-frame is likely to be longer than the athlete might expect. We, at VASTA, are in alignment with the recommendation of researchers266 that the common 6-month ‘estimate’ is too aggressive (or optimistic) for most athletes, especially younger athletes, or those with ‘extra’ risk factors described above. A 9-12 month plus expectation, in our opinion, is likely to become much more common in the near future, as clinicians begin to get a better appreciation for the complexities of advanced rehabilitation strategies necessary to break the pattern of high re-injury rates, that we are currently seeing.
Recommendations for parents of youth athletes recovering from ACLR
Expressions that are viewed by the youth athlete as excessively hesitant/cautious or “overly sympathetic” are perceived negatively by recovering athletes.267 We recommend a balance between realistic goals, an appreciation for the significance of the injury, an honest discussion around the hard work needed on their end to recover as best as possible, and some healthy optimism.
Youth athletes are also negatively impacted by a perceived lack of information and/or attention from their Physical Therapist.268 If the PT does not seem interested in the patient’s ultimate goals, or fails to provide clear guidelines / expectations for what to expect from the rehab (and Return to Sport) process, this is a problem to be identified and resolved early on.
If your athlete is rehabilitating at a facility, and they do not feel as though there is enough one-on-one attention paid to them, consider a switch.
Their care should feel focused and individualized.
Conclusion
The recovery from ACL injury – both surgical and non-operative – is a large undertaking. A good understanding of that, an appreciation for the work that lies ahead, and a proper mental approach to the recovery are all beneficial.
The Sports Physical Therapists at VASTA are uniquely equipped to safely and effectively guide the injured athlete for the full spectrum of recovery – from initial injury to return of high-level performance and return to sports.
Click here to schedule an evaluation today – or call our office at 802-399-2244
- Van den Bogert, A. J., & McLean, S. G. (2007). ACL injuries: do we know the mechanisms?. Journal of Orthopaedic and Sports Physical Therapy, 37(2), A8.
- Prodromos, C. C., Han, Y., Rogowski, J., Joyce, B., & Shi, K. (2007). A meta-analysis of the incidence of anterior cruciate ligament tears as a function of gender, sport, and a knee injury–reduction regimen. Arthroscopy: The Journal of Arthroscopic & Related Surgery, 23(12), 1320-1325.
- Warner, S. J., Smith, M. V., Wright, R. W., Matava, M. J., & Brophy, R. H. (2011). Sport-specific outcomes after anterior cruciate ligament reconstruction. Arthroscopy: The Journal of Arthroscopic & Related Surgery, 27(8), 1129-1134.
- Hootman, J. M., Dick, R., & Agel, J. (2007). Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. Journal of athletic training, 42(2), 311.
- Shaw, L., Finch, C. F., & Bekker, S. (2018). Infographic: Trends in paediatric and adolescent ACL injuries. Br J Sports Med, bjsports-2017.
- Dodwell, E. R., LaMont, L. E., Green, D. W., Pan, T. J., Marx, R. G., & Lyman, S. (2014). 20 years of pediatric anterior cruciate ligament reconstruction in New York State. The American journal of sports medicine, 42(3), 675-680.
- Mall, N. A., Chalmers, P. N., Moric, M., Tanaka, M. J., Cole, B. J., Bach Jr, B. R., & Paletta Jr, G. A. (2014). Incidence and trends of anterior cruciate ligament reconstruction in the United States. The American journal of sports medicine, 42(10), 2363-2370.
- Frank, J. S., & Gambacorta, P. L. (2013). Anterior cruciate ligament injuries in the skeletally immature athlete: diagnosis and management. JAAOS-Journal of the American Academy of Orthopaedic Surgeons, 21(2), 78-87.
- Haim, A., Pritsch, T., Yosepov, L., & Arbel, R. (2006). Anterior cruciate ligament injuries. Harefuah, 145(3), 208-14.
- Griffin LY, Agel J, Albohm MJ, et al. (2000) Noncontact anterior cruciate ligament injuries: risk factors and prevention strategies. J Am Acad Orthop Surg. 8(3):141-150.
- Arendt, E., & Dick, R. (1995). Knee injury patterns among men and women in collegiate basketball and soccer: NCAA data and review of literature. The American journal of sports medicine, 23(6), 694-701.
- Arendt, E. A., Agel, J., & Dick, R. (1999). Anterior cruciate ligament injury patterns among collegiate men and women. Journal of athletic training, 34(2), 86.
- Garrick JG, Requa RK. Anterior cruciate ligament injuries in men and women: how common are they? In: Griffin LY, ed. Prevention of noncontact ACL injuries. Rosemont,IL:American Academy Orthopaedic Surgeons,2001:1-10.
- Agel, J., Arendt, E. A., & Bershadsky, B. (2005). Anterior cruciate ligament injury in national collegiate athletic association basketball and soccer: a 13-year review. The American journal of sports medicine, 33(4), 524-531.
- Beynnon, B. D., Johnson, R. J., Abate, J. A., Fleming, B. C., & Nichols, C. E. (2005). Treatment of anterior cruciate ligament injuries, part I. The American journal of sports medicine, 33(10), 1579-1602.
- Arendt, E. A. (2001). Anterior cruciate ligament injuries. Current women’s health reports, 1(3), 211-217.
- Shelbourne, K. D., Gray, T., & Haro, M. (2009). Incidence of subsequent injury to either knee within 5 years after anterior cruciate ligament reconstruction with patellar tendon autograft. The American journal of sports medicine, 37(2), 246-251.
- Paterno, M. V., Rauh, M. J., Schmitt, L. C., Ford, K. R., & Hewett, T. E. (2012). Incidence of contralateral and ipsilateral anterior cruciate ligament (ACL) injury after primary ACL reconstruction and return to sport. Clinical journal of sport medicine: official journal of the Canadian Academy of Sport Medicine, 22(2), 116.
- Geng, B., Wang, J., Ma, J. L., Zhang, B., Jiang, J., Tan, X. Y., & Xia, Y. Y. (2016). Narrow intercondylar notch and anterior cruciate ligament injury in female nonathletes with knee osteoarthritis aged 41–65 years in plateau region. Chinese medical journal, 129(21), 2540.
- McLean, S. G., Huang, X., & Van Den Bogert, A. J. (2005). Association between lower extremity posture at contact and peak knee valgus moment during sidestepping: implications for ACL injury. Clinical Biomechanics, 20(8), 863-870.
- Mountcastle, S. B., Posner, M., Kragh, J. F., & Taylor Jr, D. C. (2007). Gender differences in anterior cruciate ligament injury vary with activity: epidemiology of anterior cruciate ligament injuries in a young, athletic population. The American journal of sports medicine, 35(10), 1635-1642.
- Price, M. J., Tuca, M., Cordasco, F. A., & Green, D. W. (2017). Nonmodifiable risk factors for anterior cruciate ligament injury. Current opinion in pediatrics, 29(1), 55-64.
- Renstrom, P., Ljungqvist, A., Arendt, E., Beynnon, B., Fukubayashi, T., Garrett, W., … & Mandelbaum, B. (2008). Non-contact ACL injuries in female athletes: an International Olympic Committee current concepts statement. British journal of sports medicine, 42(6), 394-412.
- Hewett, T. E., Ford, K. R., & Myer, G. D. (2006). Anterior cruciate ligament injuries in female athletes: Part 2, a meta-analysis of neuromuscular interventions aimed at injury prevention. The American journal of sports medicine, 34(3), 490-498.
- Sutton, K. M., & Bullock, J. M. (2013). Anterior cruciate ligament rupture: differences between males and females. JAAOS-Journal of the American Academy of Orthopaedic Surgeons, 21(1), 41-50.
- Purnell, M. L., Larson, A. I., & Clancy, W. (2008). Anterior cruciate ligament insertions on the tibia and femur and their relationships to critical bony landmarks using high-resolution volume-rendering computed tomography. The American journal of sports medicine, 36(11), 2083-2090.
- Girgis, F. G., Marshall, J. L., & Monajem, A. R. S. A. (1975). The cruciate ligaments of the knee joint. Anatomical, functional and experimental analysis. Clinical orthopaedics and related research, (106), 216-231.
- Harner, C. D., Baek, G. H., Vogrin, T. M., Carlin, G. J., Kashiwaguchi, S., & Woo, S. L. (1999). Quantitative analysis of human cruciate ligament insertions. Arthroscopy: The Journal of Arthroscopic & Related Surgery, 15(7), 741-749.
- Girgis, F. G., Marshall, J. L., & Monajem, A. R. S. A. (1975). The cruciate ligaments of the knee joint. Anatomical, functional and experimental analysis. Clinical orthopaedics and related research, (106), 216-231.
- Zantop, T., Petersen, W., Sekiya, J. K., Musahl, V., & Fu, F. H. (2006). Anterior cruciate ligament anatomy and function relating to anatomical reconstruction. Knee surgery, sports traumatology, arthroscopy, 14(10), 982-992.
- Petersen, W., & Tillmann, B. (1999). Structure and vascularization of the cruciate ligaments of the human knee joint. Anatomy and embryology, 200(3), 325-334.
- Haus, J., & Halata, Z. (1990). Innervation of the anterior cruciate ligament. International orthopaedics, 14(3), 293-296.
- Kennedy, J. C., Alexander, I. J., & Hayes, K. C. (1982). Nerve supply of the human knee and its functional importance. The American journal of sports medicine, 10(6), 329-335.
- Hogervorst, T., & Brand, R. A. (1998). Current concepts review-mechanoreceptors in joint function. JBJS, 80(9), 1365-1378.
- Konishi, Y., Fukubayashi, T., & Takeshita, D. (2002). Possible mechanism of quadriceps femoris weakness in patients with ruptured anterior cruciate ligament. Medicine and science in sports and exercise, 34(9), 1414-1418.
- Girgis, F. G., Marshall, J. L., & Monajem, A. R. S. A. (1975). The cruciate ligaments of the knee joint. Anatomical, functional and experimental analysis. Clinical orthopaedics and related research, (106), 216-231.
- [ReadySetMed]. (2014, October 5). What is the ACL and how does it work? ACL Series. [Video file]. Retrieved from https://www.youtube.com/watch?v=-3ZYNV0Py5E
- Petersen, W., & Zantop, T. (2007). Anatomy of the anterior cruciate ligament with regard to its two bundles. Clinical Orthopaedics and Related Research, 454, 35-47.
- Matsumoto, H., Suda, Y., Otani, T., Niki, Y., Seedhom, B. B., & Fujikawa, K. (2001). Roles of the anterior cruciate ligament and the medial collateral ligament in preventing valgus instability. Journal of orthopaedic science, 6(1), 28-32.
- [ReadySetMed]. (2014, October 5). What Role Does the ACL Play in Sports? ACL Series. [Video file]. Retrieved from https://www.youtube.com/watch?v=_4HJqBtlsEs
- Hewett, T. E., Myer, G. D., & Ford, K. R. (2006). Anterior cruciate ligament injuries in female athletes: Part 1, mechanisms and risk factors. The American journal of sports medicine, 34(2), 299-311.
- Haim, A., Pritsch, T., Yosepov, L., & Arbel, R. (2006). Anterior cruciate ligament injuries. Harefuah, 145(3), 208-14.
- Neumann, Donald. Kinesiology of the Musculoskeletal System: Foundations for Rehabilitation. 2nd edition. St. Louis, MO: Mosby Elsevier, 2010. 535. Print.
- Olsen, O. E., Myklebust, G., Engebretsen, L., & Bahr, R. (2004). Injury mechanisms for anterior cruciate ligament injuries in team handball: a systematic video analysis. The American journal of sports medicine, 32(4), 1002-1012.
- Teitz CC.video analysis of ACL injuries. In:Griffin LY, ed. Prevention of Non contact ACL injuries. Rosemont,IL: American Academy Orthopaedic Surgeons,2001
- Koga, H., Nakamae, A., Shima, Y., Iwasa, J., Myklebust, G., Engebretsen, L., … & Krosshaug, T. (2010). Mechanisms for non-contact anterior cruciate ligament injuries: knee joint kinematics in 10 injury situations from female team handball and basketball. The American journal of sports medicine, 38(11), 2218-2225.
- Teitz CC.video analysis of ACL injuries. In:Griffin LY, ed. Prevention of Non contact ACL injuries.Rosemont,IL: American Academy Orthopaedic Surgeons,2001
- Prentice, W. E. (2004). Rehabilitation techniques for sports medicine and athletic training.
- Souryal, T. O., & Freeman, T. R. (1993). Intercondylar notch size and anterior cruciate ligament injuries in athletes: a prospective study. The American journal of sports medicine, 21(4), 535-539
- Longo, U. G., Loppini, M., Berton, A., Marinozzi, A., Maffulli, N., & Denaro, V. (2012). The FIFA 11+ program is effective in preventing injuries in elite male basketball players: a cluster randomized controlled trial. The American journal of sports medicine, 40(5), 996-1005.
- Mandelbaum, B. R., Silvers, H. J., Watanabe, D. S., Knarr, J. F., Thomas, S. D., Griffin, L. Y., … & Garrett Jr, W. (2005). Effectiveness of a neuromuscular and proprioceptive training program in preventing anterior cruciate ligament injuries in female athletes: 2-year follow-up. The American journal of sports medicine, 33(7), 1003-1010.
- Steffen, K., Emery, C. A., Romiti, M., Kang, J., Bizzini, M., Dvorak, J., … & Meeuwisse, W. H. (2013). High adherence to a neuromuscular injury prevention programme (FIFA 11+) improves functional balance and reduces injury risk in Canadian youth female football players: a cluster randomised trial. Br J Sports Med, bjsports-2012.
- Hewett, T. E., Ford, K. R., Hoogenboom, B. J., & Myer, G. D. (2010). Understanding and preventing acl injuries: current biomechanical and epidemiologic considerations-update 2010. North American journal of sports physical therapy: NAJSPT, 5(4), 234.
- Brukne, Khan. Clinical Sports Medicine. 3rd edition.Ch 27.Tata McGraw- Hill Publishing. New Delhi
- Landis, S. E., Baker, R. T., & Seegmiller, J. G. (2018). Non-Contact Anterior Cruciate Ligament and Lower Extremity Injury Risk Prediction Using Functional Movement Screen and Knee Abduction Moment: An Epidemiological observation of Female Intercollegiate Athletes. International journal of sports physical therapy, 13(6), 973.
- Yoon, K. H., Yoo, J. H., & Kim, K. I. (2011). Bone contusion and associated meniscal and medial collateral ligament injury in patients with anterior cruciate ligament rupture. JBJS, 93(16), 1510-1518.
- Rosen, M. A., Jackson, D. W., & Berger, P. E. (1992). Occult osseous lesions documented by magnetic resonance imaging associated with anterior cruciate ligament ruptures. Clinical Journal of Sport Medicine, 2(1), 74.
- Frobell, R. B., Roos, H. P., Roos, E. M., Le Graverand, M. P. H., Buck, R., Tamez-Pena, J., … & Lohmander, L. S. (2008). The acutely ACL injured knee assessed by MRI: are large volume traumatic bone marrow lesions a sign of severe compression injury? Osteoarthritis and Cartilage, 16(7), 829-836.
- Niall, D. M., Bobic, V., Surgeon, C. O. K., & Lodge, N. (2004). Bone bruising and bone marrow edema syndromes: incidental radiological findings or harbingers of future joint degeneration. International Society of Arthroscopy, Knee Surgery and Orthopaedic Sports Medicine
- Yoon, K. H., Yoo, J. H., & Kim, K. I. (2011). Bone contusion and associated meniscal and medial collateral ligament injury in patients with anterior cruciate ligament rupture. JBJS, 93(16), 1510-1518.
- Szkopek, K., Warming, T., Neergaard, K., Jørgensen, H. L., Christensen, H. E., & Krogsgaard, M. (2012). Pain and knee function in relation to degree of bone bruise after acute anterior cruciate ligament rupture. Scandinavian journal of medicine & science in sports, 22(5), 635-642.
- Papalia, R., Torre, G., Vasta, S., Zampogna, B., Pedersen, D. R., Denaro, V., & Amendola, A. (2015). Bone bruises in anterior cruciate ligament injured knee and long-term outcomes. A review of the evidence. Open access journal of sports medicine, 6, 37.
- Nakamae, A., Engebretsen, L., Bahr, R., Krosshaug, T., & Ochi, M. (2006). Natural history of bone bruises after acute knee injury: clinical outcome and histopathological findings. Knee Surgery, Sports Traumatology, Arthroscopy, 14(12), 1252-1258.
- Papalia, R., Torre, G., Vasta, S., Zampogna, B., Pedersen, D. R., Denaro, V., & Amendola, A. (2015). Bone bruises in anterior cruciate ligament injured knee and long-term outcomes. A review of the evidence. Open access journal of sports medicine, 6, 37.
- Dare, D. M., Fabricant, P. D., McCarthy, M. M., Rebolledo, B. J., Green, D. W., Cordasco, F. A., & Jones, K. J. (2015). Increased lateral tibial slope is a risk factor for pediatric anterior cruciate ligament injury: an MRI-based case-control study of 152 patients. The American journal of sports medicine, 43(7), 1632-1639.
- Todd, M. S., Lalliss, S., Garcia, E. S., DeBerardino, T. M., & Cameron, K. L. (2010). The relationship between posterior tibial slope and anterior cruciate ligament injuries. The American journal of sports medicine, 38(1), 63-67.
- Waiwaiole, A., Gurbani, A., Motamedi, K., Seeger, L., Sim, M. S., Nwajuaku, P., & Hame, S. L. (2016). Relationship of ACL injury and posterior tibial slope with patient age, sex, and race. Orthopaedic Journal of Sports Medicine, 4(11), 2325967116672852.
- Wang, Y. L., Yang, T., Zeng, C., Wei, J., Xie, D. X., Yang, Y. H., … & Lei, G. H. (2017). Association between tibial plateau slopes and anterior cruciate ligament injury: a meta-analysis. Arthroscopy: The Journal of Arthroscopic & Related Surgery, 33(6), 1248-1259.
- Webb, J. M., Salmon, L. J., Leclerc, E., Pinczewski, L. A., & Roe, J. P. (2013). Posterior tibial slope and further anterior cruciate ligament injuries in the anterior cruciate ligament–reconstructed patient. The American journal of sports medicine, 41(12), 2800-2804.
- Feucht, M. J., Mauro, C. S., Brucker, P. U., Imhoff, A. B., & Hinterwimmer, S. (2013). The role of the tibial slope in sustaining and treating anterior cruciate ligament injuries. Knee surgery, sports traumatology, arthroscopy, 21(1), 134-145.
- Christensen, J. J., Krych, A. J., Engasser, W. M., Vanhees, M. K., Collins, M. S., & Dahm, D. L. (2015). Lateral tibial posterior slope is increased in patients with early graft failure after anterior cruciate ligament reconstruction. The American journal of sports medicine, 43(10), 2510-2514.
- Kiapour, A. M., Yang, D. S., Badger, G. J., Karamchedu, N. P., Murray, M. M., Fadale, P. D., … & Fleming, B. C. (2019). Anatomic Features of the Tibial Plateau Predict Outcomes of ACL Reconstruction Within 7 Years After Surgery. The American journal of sports medicine, 0363546518823556.
- Salmon, L. J., Heath, E., Akrawi, H., Roe, J. P., Linklater, J., & Pinczewski, L. A. (2018). 20-Year outcomes of anterior cruciate ligament reconstruction with hamstring tendon autograft: the catastrophic effect of age and posterior tibial slope. The American journal of sports medicine, 46(3), 531-543.
- Thomson, A., Whiteley, R., & Bleakley, C. (2015). Higher shoe-surface interaction is associated with doubling of lower extremity injury risk in football codes: a systematic review and meta-analysis. Br J Sports Med, 49(19), 1245-1252.
- Lambson, R. B., Barnhill, B. S., & Higgins, R. W. (1996). Football cleat design and its effect on anterior cruciate ligament injuries: a three-year prospective study. The American journal of sports medicine, 24(2), 155-159.
- Orchard, J. (2001). The AFL penetrometer study: work in progress. Journal of Science and Medicine in Sport, 4(2), 220-232.
- Olsen, O. E., Myklebust, G., Engebretsen, L., Holme, I., & Bahr, R. (2003). Relationship between floor type and risk of ACL injury in team handball. Scandinavian journal of medicine & science in sports, 13(5), 299-304.
- Ireland ML.Anterior cruciate ligament injuries in young female athletes.Your Patient and Fitness 1996;10(5):26-30.
- Teitz CC.video analysis of ACL injuries. In:Griffin LY, ed. Prevention of Non contact ACL injuries.Rosemont,IL: American Academy Orthopaedic Surgeons,2001
- Renstrom, P., Ljungqvist, A., Arendt, E., Beynnon, B., Fukubayashi, T., Garrett, W., … & Mandelbaum, B. (2008). Non-contact ACL injuries in female athletes: an International Olympic Committee current concepts statement. British journal of sports medicine, 42(6), 394-412.
- Paterno, M. V., Schmitt, L. C., Ford, K. R., Rauh, M. J., Myer, G. D., Huang, B., & Hewett, T. E. (2010). Biomechanical measures during landing and postural stability predict second anterior cruciate ligament injury after anterior cruciate ligament reconstruction and return to sport. The American journal of sports medicine, 38(10), 1968-1978.
- Nagai, T., Heebner, N. R., Sell, T. C., Nakagawa, T., Fu, F. H., & Lephart, S. M. (2013). Restoration of sagittal and transverse plane proprioception following anatomic double-bundle ACL reconstruction. Knee surgery, sports traumatology, arthroscopy, 21(9), 2048-2056.
- Paterno, M. V., Schmitt, L. C., Ford, K. R., Rauh, M. J., Myer, G. D., Huang, B., & Hewett, T. E. (2010). Biomechanical measures during landing and postural stability predict second anterior cruciate ligament injury after anterior cruciate ligament reconstruction and return to sport. The American journal of sports medicine, 38(10), 1968-1978.
- Kiefer, A. W., Ford, K. R., Paterno, M. V., Schmitt, L. C., Myer, G. D., Riley, M. A., … & Hewett, T. E. (2013). Inter-segmental postural coordination measures differentiate athletes with ACL reconstruction from uninjured athletes. Gait & posture, 37(2), 149-153.
- Hewett, T. E., Ford, K. R., & Myer, G. D. (2006). Anterior cruciate ligament injuries in female athletes: Part 2, a meta-analysis of neuromuscular interventions aimed at injury prevention. The American journal of sports medicine, 34(3), 490-498.
- Hewett, T. E., Myer, G. D., Ford, K. R., Heidt Jr, R. S., Colosimo, A. J., McLean, S. G., … & Succop, P. (2005). Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. The American journal of sports medicine, 33(4), 492-501.
- Paterno, M. V., Schmitt, L. C., Ford, K. R., Rauh, M. J., Myer, G. D., Huang, B., & Hewett, T. E. (2010). Biomechanical measures during landing and postural stability predict second anterior cruciate ligament injury after anterior cruciate ligament reconstruction and return to sport. The American journal of sports medicine, 38(10), 1968-1978.
- Kiefer, A. W., Ford, K. R., Paterno, M. V., Schmitt, L. C., Myer, G. D., Riley, M. A., … & Hewett, T. E. (2013). Inter-segmental postural coordination measures differentiate athletes with ACL reconstruction from uninjured athletes. Gait & posture, 37(2), 149-153.
- Myer, G. D., Ford, K. R., Brent, J. L., & Hewett, T. E. (2012). An integrated approach to change the outcome part II: targeted neuromuscular training techniques to reduce identified ACL injury risk factors. Journal of strength and conditioning research/National Strength & Conditioning Association, 26(8), 2272.
- Hewett, T. E., Myer, G. D., Ford, K. R., Heidt Jr, R. S., Colosimo, A. J., McLean, S. G., … & Succop, P. (2005). Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. The American journal of sports medicine, 33(4), 492-501.
- Bach, B. R., Levy, M. E., Bojchuk, J., Tradonsky, S., Bush-Joseph, C. A., & Khan, N. H. (1998). Single-incision endoscopic anterior cruciate ligament reconstruction using patellar tendon autograft. The American journal of sports medicine, 26(1), 30-40.
- Frank, J. S., & Gambacorta, P. L. (2013). Anterior cruciate ligament injuries in the skeletally immature athlete: diagnosis and management. JAAOS-Journal of the American Academy of Orthopaedic Surgeons, 21(2), 78-87.
- Sommerfeldt, M., Goodine, T., Raheem, A., Whittaker, J., & Otto, D. (2018). Relationship Between Time to ACL Reconstruction and Presence of Adverse Changes in the Knee at the Time of Reconstruction. Orthopaedic journal of sports medicine, 6(12), 2325967118813917.
- Ahldén, M., Samuelsson, K., Sernert, N., Forssblad, M., Karlsson, J., & Kartus, J. (2012). The Swedish National Anterior Cruciate Ligament Register: a report on baseline variables and outcomes of surgery for almost 18,000 patients. The American journal of sports medicine, 40(10), 2230-2235.
- Gifstad, T., Foss, O. A., Engebretsen, L., Lind, M., Forssblad, M., Albrektsen, G., & Drogset, J. O. (2014). Lower risk of revision with patellar tendon autografts compared with hamstring autografts: a registry study based on 45,998 primary ACL reconstructions in Scandinavia. The American journal of sports medicine, 42(10), 2319-2328.
- Kaeding, C. C., Pedroza, A. D., Reinke, E. K., Huston, L. J., Moon Consortium, & Spindler, K. P. (2015). Risk factors and predictors of subsequent ACL injury in either knee after ACL reconstruction: prospective analysis of 2488 primary ACL reconstructions from the MOON cohort. The American journal of sports medicine, 43(7), 1583-1590.
- Maletis, G. B., Chen, J., Inacio, M. C., & Funahashi, T. T. (2016). Age-related risk factors for revision anterior cruciate ligament reconstruction: a cohort study of 21,304 patients from the Kaiser Permanente Anterior Cruciate Ligament Registry. The American journal of sports medicine, 44(2), 331-336.
- Parkinson, B., Robb, C., Thomas, M., Thompson, P., & Spalding, T. (2017). Factors that predict failure in anatomic single-bundle anterior cruciate ligament reconstruction. The American journal of sports medicine, 45(7), 1529-1536.
- Wiggins, A. J., Grandhi, R. K., Schneider, D. K., Stanfield, D., Webster, K. E., & Myer, G. D. (2016). Risk of secondary injury in younger athletes after anterior cruciate ligament reconstruction: a systematic review and meta-analysis. The American journal of sports medicine, 44(7), 1861-1876.
- Kaplan, Y. (2011). Identifying individuals with an anterior cruciate ligament-deficient knee as copers and noncopers: a narrative literature review. Journal of orthopaedic & sports physical therapy, 41(10), 758-766.
- Caborn, D. N., & Johnson, B. M. (1993). The natural history of the anterior cruciate ligament-deficient knee. A review. Clinics in sports medicine, 12(4), 625-636.
- Fitzgerald, G. K., Axe, M. J., & Snyder-Mackler, L. (2000). A decision-making scheme for returning patients to high-level activity with nonoperative treatment after anterior cruciate ligament rupture. Knee Surgery, Sports Traumatology, Arthroscopy, 8(2), 76-82.
- Hewett, T. E., Blum, K. R., & Noyes, F. R. (1997). Gait characteristics of the anterior cruciate ligament-deficient varus knee. The American journal of knee surgery, 10(4), 246.
- Johnson, D. H., Maffulli, N., King, J. B., & Shelbourne, K. D. (2003). Anterior cruciate ligament reconstruction: a cynical view from the British Isles on the indications for surgery. Arthroscopy: The Journal of Arthroscopic & Related Surgery, 19(2), 203-209.
- Rudolph, K. S., Axe, M. J., & Snyder-Mackler, L. (2000). Dynamic stability after ACL injury: who can hop?. Knee Surgery, Sports Traumatology, Arthroscopy, 8(5), 262-269.
- Walla, D. J., Albright, J. P., McAuley, E., Martin, R. K., Eldridge, V., & El-Khoury, G. (1985). Hamstring control and the unstable anterior cruciate ligament-deficient knee. The American journal of sports medicine, 13(1), 34-39.
- Daniel, D. M., Stone, M. L., Sachs, R., & Malcom, L. (1985). Instrumented measurement of anterior knee laxity in patients with acute anterior cruciate ligament disruption. The American journal of sports medicine, 13(6), 401-407.
- Kaplan, Y. (2011). Identifying individuals with an anterior cruciate ligament-deficient knee as copers and noncopers: a narrative literature review. Journal of orthopaedic & sports physical therapy, 41(10), 758-766.
- Levins, J. G., Argentieri, E. C., Sturnick, D. R., Gardner-Morse, M., Vacek, P. M., Tourville, T. W., … & Beynnon, B. D. (2017). Geometric Characteristics of the Knee Are Associated With a Noncontact ACL Injury to the Contralateral Knee After Unilateral ACL Injury in Young Female Athletes. The American journal of sports medicine, 45(14), 3223-3232.
- Levins, J. G., Sturnick, D. R., Argentieri, E. C., Gardner-Morse, M., Vacek, P. M., Desarno, M. J., … & Beynnon, B. D. (2016). Geometric risk factors associated with noncontact anterior cruciate ligament graft rupture. The American journal of sports medicine, 44(10), 2537-2545.
- Brukne, Khan. Clinical Sports Medicine. 3rd edition.Ch 27.Tata McGraw- Hill Publishing. New Delhi
- Kostogiannis, I., Ageberg, E., Neuman, P., Dahlberg, L., Friden, T., & Roos, H. (2007). Activity level and subjective knee function 15 years after anterior cruciate ligament injury: a prospective, longitudinal study of nonreconstructed patients. The American journal of sports medicine, 35(7), 1135-1143.
- Hurd, W. J., Axe, M. J., & Snyder-Mackler, L. (2008). A 10-year prospective trial of a patient management algorithm and screening examination for highly active individuals with anterior cruciate ligament injury: part 2, determinants of dynamic knee stability. The American journal of sports medicine, 36(1), 48-56.
- Alkjær, T., Henriksen, M., & Simonsen, E. B. (2011). Different knee joint loading patterns in ACL deficient copers and non-copers during walking. Knee Surgery, Sports Traumatology, Arthroscopy, 19(4), 615-621.
- Anderson, A. F., Irrgang, J. J., Kocher, M. S., Mann, B. J., Harrast, J. J., & International Knee Documentation Committee. (2006). The International Knee Documentation Committee subjective knee evaluation form: normative data. The American journal of sports medicine, 34(1), 128-135.
- Button, K., van Deursen, R., & Price, P. (2008). Recovery in functional non-copers following anterior cruciate ligament rupture as detected by gait kinematics. Physical Therapy in Sport, 9(2), 97-104.
- Chmielewski, T. L., Rudolph, K. S., Fitzgerald, G. K., Axe, M. J., & Snyder-Mackler, L. (2001). Biomechanical evidence supporting a differential response to acute ACL injury. Clinical biomechanics, 16(7), 586-591.
- Eastlack M.E, Axe M.J, Snyder-Mackler L.. (1999) Laxity, instability, and functional outcome after ACL injury; copers versus noncopers. Med Sci Sports Exerc, 31(2):210-215.
- Herrington, L., & Fowler, E. (2006). A systematic literature review to investigate if we identify those patients who can cope with anterior cruciate ligament deficiency. The Knee, 13(4), 260-265.
- Houck, J. R., De Haven, K. E., & Maloney, M. (2007). Influence of anticipation on movement patterns in subjects with ACL deficiency classified as noncopers. Journal of Orthopaedic & Sports Physical Therapy, 37(2), 56-64.
- Moksnes, H., Snyder-Mackler, L., & Risberg, M. A. (2008). Individuals with an anterior cruciate ligament-deficient knee classified as noncopers may be candidates for nonsurgical rehabilitation. Journal of Orthopaedic & Sports Physical Therapy, 38(10), 586-595.
- Sandberg, R., Balkfors, B., Nilsson, B., & Westlin, N. (1987). Operative versus non-operative treatment of recent injuries to the ligaments of the knee. A prospective randomized study. The Journal of bone and joint surgery. American volume, 69(8), 1120-1126.
- Shea, K. G., Carey, J. L., Richmond, J., Sandmeier, R., Pitts, R. T., Polousky, J. D., … & LaBella, C. R. (2015). The American Academy of Orthopaedic Surgeons evidence-based guideline on management of anterior cruciate ligament injuries. JBJS, 97(8), 672-674.
- Andersson, C., Odensten, M., & Gillquist, J. (1991). Knee function after surgical or nonsurgical treatment of acute rupture of the anterior cruciate ligament: a randomized study with a long-term follow-up period. Clinical orthopaedics and related research, (264), 255-263.
- Barrack, R. L., Bruckner, J. D., Kneisl, J., Inman, W. S., & Alexander, A. H. (1990). The outcome of nonoperatively treated complete tears of the anterior cruciate ligament in active young adults. Clinical orthopaedics and related research, (259), 192-199.
- Hawkins, R. J., Misamore, G. W., & Merritt, T. R. (1986). Followup of the acute nonoperated isolated anterior cruciate ligament tear. The American journal of sports medicine, 14(3), 205-210.
- Jones, H. P., Appleyard, R. C., Mahajan, S., & Murrell, G. A. (2003). Meniscal and chondral loss in the anterior cruciate ligament injured knee. Sports Medicine, 33(14), 1075-1089.
- Kannus, P., & Järvinen, M. (1987). Conservatively treated tears of the anterior cruciate ligament. Long-term results. JBJS, 69(7), 1007-1012. http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.914.1220&rep=rep1&type=pdf
- O’neill, D. B. (1996). Arthroscopically Assisted Reconstruction of the Anterior Cruciate Ligament.: A Prospective Randomized Analysis of Three Techniques. JBJS, 78(6), 803-813.
- Smith, F. W., Rosenlund, E. A., Aune, A. K., MacLean, J. A., & Hillis, S. W. (2004). Subjective functional assessments and the return to competitive sport after anterior cruciate ligament reconstruction. British journal of sports medicine, 38(3), 279-284.
- Shah, V. M., Andrews, J. R., Fleisig, G. S., McMichael, C. S., & Lemak, L. J. (2010). Return to play after anterior cruciate ligament reconstruction in National Football League athletes. The American journal of sports medicine, 38(11), 2233-2239.
- Read, C. R., Aune, K. T., Cain Jr, E. L., & Fleisig, G. S. (2017). Return to play and decreased performance after anterior cruciate ligament reconstruction in National Football League defensive players. The American journal of sports medicine, 45(8), 1815-1821.
- Ardern, C. L. (2015). Anterior cruciate ligament reconstruction—not exactly a one-way ticket back to the preinjury level: a review of contextual factors affecting return to sport after surgery. Sports health, 7(3), 224-230.
- Bell, D. R., Pfeiffer, K. A., Cadmus-Bertram, L. A., Trigsted, S. M., Kelly, A., Post, E. G., … & Kuenze, C. (2017). Objectively measured physical activity in patients after anterior cruciate ligament reconstruction. The American journal of sports medicine, 45(8), 1893-1900.
- Ardern, C. L., Webster, K. E., Taylor, N. F., & Feller, J. A. (2011). Return to the preinjury level of competitive sport after anterior cruciate ligament reconstruction surgery: two-thirds of patients have not returned by 12 months after surgery. The American journal of sports medicine, 39(3), 538-543.
- Hartigan, E. H., Axe, M. J., & Snyder-Mackler, L. (2010). Time line for noncopers to pass return-to-sports criteria after anterior cruciate ligament reconstruction. Journal of orthopaedic & sports physical therapy, 40(3), 141-154.
- Ardern, C. L., Taylor, N. F., Feller, J. A., & Webster, K. E. (2014). Fifty-five per cent return to competitive sport following anterior cruciate ligament reconstruction surgery: an updated systematic review and meta-analysis including aspects of physical functioning and contextual factors. Br J Sports Med, 48(21), 1543-1552.
- Ardern, C. L., Webster, K. E., Taylor, N. F., & Feller, J. A. (2011). Return to the preinjury level of competitive sport after anterior cruciate ligament reconstruction surgery: two-thirds of patients have not returned by 12 months after surgery. The American journal of sports medicine, 39(3), 538-543.
- Ardern, C. L., Taylor, N. F., Feller, J. A., & Webster, K. E. (2014). Fifty-five per cent return to competitive sport following anterior cruciate ligament reconstruction surgery: an updated systematic review and meta-analysis including aspects of physical functioning and contextual factors. Br J Sports Med, 48(21), 1543-1552.
- Ardern, C. L., Webster, K. E., Taylor, N. F., & Feller, J. A. (2011). Return to the preinjury level of competitive sport after anterior cruciate ligament reconstruction surgery: two-thirds of patients have not returned by 12 months after surgery. The American journal of sports medicine, 39(3), 538-543.
- Langford, J. L., Webster, K. E., & Feller, J. A. (2009). A prospective longitudinal study to assess psychological changes following anterior cruciate ligament reconstruction surgery. British journal of sports medicine, 43(5), 377-378.
- Ardern, C. L., Taylor, N. F., Feller, J. A., & Webster, K. E. (2012). Return-to-sport outcomes at 2 to 7 years after anterior cruciate ligament reconstruction surgery. The American journal of sports medicine, 40(1), 41-48.
- Webster, K. E., Feller, J. A., Kimp, A. J., & Whitehead, T. S. (2018). Low rates of return to preinjury sport after bilateral anterior cruciate ligament reconstruction. The American journal of sports medicine, 0363546518813901.
- Wright, R. W., Gill, C. S., Chen, L., Brophy, R. H., Matava, M. J., Smith, M. V., & Mall, N. A. (2012). Outcome of revision anterior cruciate ligament reconstruction: a systematic review. The Journal of Bone and Joint Surgery. American volume., 94(6), 531.
- Sonesson, S., Kvist, J., Ardern, C., Österberg, A., & Silbernagel, K. G. (2017). Psychological factors are important to return to pre-injury sport activity after anterior cruciate ligament reconstruction: expect and motivate to satisfy. Knee surgery, sports traumatology, arthroscopy, 25(5), 1375-1384.
- Ardern, C. L., Taylor, N. F., Feller, J. A., Whitehead, T. S., & Webster, K. E. (2015). Sports participation 2 years after anterior cruciate ligament reconstruction in athletes who had not returned to sport at 1 year: a prospective follow-up of physical function and psychological factors in 122 athletes. The American journal of sports medicine, 43(4), 848-856.
- Ardern, C. L., Taylor, N. F., Feller, J. A., & Webster, K. E. (2014). Fifty-five per cent return to competitive sport following anterior cruciate ligament reconstruction surgery: an updated systematic review and meta-analysis including aspects of physical functioning and contextual factors. Br J Sports Med, 48(21), 1543-1552.
- Ardern, C. L., Taylor, N. F., Feller, J. A., Whitehead, T. S., & Webster, K. E. (2013). Psychological responses matter in returning to preinjury level of sport after anterior cruciate ligament reconstruction surgery. The American journal of sports medicine, 41(7), 1549-1558.
- Ardern, C. L., Taylor, N. F., Feller, J. A., & Webster, K. E. (2012). Return-to-sport outcomes at 2 to 7 years after anterior cruciate ligament reconstruction surgery. The American journal of sports medicine, 40(1), 41-48.
- Langford, J. L., Webster, K. E., & Feller, J. A. (2009). A prospective longitudinal study to assess psychological changes following anterior cruciate ligament reconstruction surgery. British journal of sports medicine, 43(5), 377-378.
- Lai, C. C., Ardern, C. L., Feller, J. A., & Webster, K. E. (2018). Eighty-three per cent of elite athletes return to preinjury sport after anterior cruciate ligament reconstruction: a systematic review with meta-analysis of return to sport rates, graft rupture rates and performance outcomes. Br J Sports Med, 52(2), 128-138.
- Sonesson, S., Kvist, J., Ardern, C., Österberg, A., & Silbernagel, K. G. (2017). Psychological factors are important to return to pre-injury sport activity after anterior cruciate ligament reconstruction: expect and motivate to satisfy. Knee surgery, sports traumatology, arthroscopy, 25(5), 1375-1384.
- Ardern, C. L., Taylor, N. F., Feller, J. A., Whitehead, T. S., & Webster, K. E. (2015). Sports participation 2 years after anterior cruciate ligament reconstruction in athletes who had not returned to sport at 1 year: a prospective follow-up of physical function and psychological factors in 122 athletes. The American journal of sports medicine, 43(4), 848-856.
- Ardern, C. L., Taylor, N. F., Feller, J. A., Whitehead, T. S., & Webster, K. E. (2015). Sports participation 2 years after anterior cruciate ligament reconstruction in athletes who had not returned to sport at 1 year: a prospective follow-up of physical function and psychological factors in 122 athletes. The American journal of sports medicine, 43(4), 848-856.
- Lai, C. C., Ardern, C. L., Feller, J. A., & Webster, K. E. (2018). Eighty-three per cent of elite athletes return to preinjury sport after anterior cruciate ligament reconstruction: a systematic review with meta-analysis of return to sport rates, graft rupture rates and performance outcomes. Br J Sports Med, 52(2), 128-138.
- Aglioti, S. M., Cesari, P., Romani, M., & Urgesi, C. (2008). Action anticipation and motor resonance in elite basketball players. Nature neuroscience, 11(9), 1109.
- Lorenz, D. S., Reiman, M. P., Lehecka, B. J., & Naylor, A. (2013). What performance characteristics determine elite versus nonelite athletes in the same sport? Sports health, 5(6), 542-547.
- Lin, C. H., Lien, Y. H., Wang, S. F., & Tsauo, J. Y. (2006). Hip and knee proprioception in elite, amateur, and novice tennis players. American journal of physical medicine & rehabilitation, 85(3), 216-221.
- Muaidi, Q. I., Nicholson, L. L., & Refshauge, K. M. (2009). Do elite athletes exhibit enhanced proprioceptive acuity, range and strength of knee rotation compared with non‐athletes? Scandinavian journal of medicine & science in sports, 19(1), 103-112.
- Marsh, H. W., Perry, C., Horsely, C., & Roche, L. (1995). Multidimensional self-concepts of elite athletes: How do they differ from the general population? Journal of sport and exercise Psychology, 17(1), 70-83.
- Koning, R. H., & Amelink, R. (2012). Medium-term mortality of Dutch professional soccer players.
- Lai, C. C., Ardern, C. L., Feller, J. A., & Webster, K. E. (2018). Eighty-three per cent of elite athletes return to preinjury sport after anterior cruciate ligament reconstruction: a systematic review with meta-analysis of return to sport rates, graft rupture rates and performance outcomes. Br J Sports Med, 52(2), 128-138.
- [imsportsvideos]. (2011, December 28). ACL Surgery – 3D Reconstruction. [Video File] Retrieved from https://www.youtube.com/watch?v=Xsq0sQp6DwU
- Wilson, T. W., Zafuta, M. P., & Zobitz, M. (1999). A biomechanical analysis of matched bone-patellar tendon-bone and double-looped semitendinosus and gracilis tendon grafts. The American journal of sports medicine, 27(2), 202-207.
- Pan, X., Wen, H., Wang, L., & Ge, T. (2013). Bone–patellar tendon–bone autograft versus LARS artificial ligament for anterior cruciate ligament reconstruction. European Journal of Orthopaedic Surgery & Traumatology, 23(7), 819-823.
- Manske, R. C. (2006). Postsurgical orthopedic sports rehabilitation: knee & shoulder. Elsevier Health Sciences. 176-177, 191-192
- Zelić, Z., Jovanović, S., Wertheimer, V., Šarić, G., Biuk, E., & Gulan, G. (2012). Results of the surgical reconstruction of the anterior cruciate ligament. Collegium antropologicum, 36(1), 201-206.
- Abbas, M. M., Abulaban, A. A., & Darwish, H. H. (2013). Functional outcomes of bone tendon bone versus soft tissue arthroscopic anterior cruciate ligament reconstruction. A comparative study. Saudi Medical Journal, 34(2).
- Zelić, Z., Jovanović, S., Wertheimer, V., Šarić, G., Biuk, E., & Gulan, G. (2012). Results of the surgical reconstruction of the anterior cruciate ligament. Collegium antropologicum, 36(1), 201-206.
- Abbas, M. M., Abulaban, A. A., & Darwish, H. H. (2013). Functional outcomes of bone tendon bone versus soft tissue arthroscopic anterior cruciate ligament reconstruction. A comparative study. Saudi Medical Journal, 34(2).
- Wilson, T. W., Zafuta, M. P., & Zobitz, M. (1999). A biomechanical analysis of matched bone-patellar tendon-bone and double-looped semitendinosus and gracilis tendon grafts. The American journal of sports medicine, 27(2), 202-207.
- Xu, M., Gao, S., Zeng, C., Han, R., Sun, J., Li, H., … & Lei, G. (2013). Outcomes of anterior cruciate ligament reconstruction using single-bundle versus double-bundle technique: meta-analysis of 19 randomized controlled trials. Arthroscopy: The Journal of Arthroscopic & Related Surgery, 29(2), 357-365. https://www.sciencedirect.com/science/article/pii/S0749806312016994
- Tashman, S., Kopf, S., & Fu, F. H. (2012). The kinematic basis of anterior cruciate ligament reconstruction. Operative techniques in sports medicine, 20(1), 19-22.
- Siebold, R., Dehler, C., & Ellert, T. (2008). Prospective randomized comparison of double-bundle versus single-bundle anterior cruciate ligament reconstruction. Arthroscopy: The Journal of Arthroscopic & Related Surgery, 24(2), 137-145.
- Cinque, M. E., Dornan, G. J., Chahla, J., Moatshe, G., & LaPrade, R. F. (2017). High rates of osteoarthritis develop after anterior cruciate ligament surgery: an analysis of 4108 patients. The American journal of sports medicine, 0363546517730072.
- Frank, J. S., & Gambacorta, P. L. (2013). Anterior cruciate ligament injuries in the skeletally immature athlete: diagnosis and management. JAAOS-Journal of the American Academy of Orthopaedic Surgeons, 21(2), 78-87.
- Magnussen, R. A., Pedroza, A. D., Donaldson, C. T., Flanigan, D. C., & Kaeding, C. C. (2013). Time from ACL injury to reconstruction and the prevalence of additional intra-articular pathology: is patient age an important factor?. Knee Surgery, Sports Traumatology, Arthroscopy, 21(9), 2029-2034.
- Frank, J. S., & Gambacorta, P. L. (2013). Anterior cruciate ligament injuries in the skeletally immature athlete: diagnosis and management. JAAOS-Journal of the American Academy of Orthopaedic Surgeons, 21(2), 78-87.
- Joreitz, R., Lynch, A., Rabuck, S., Lynch, B., Davin, S., & Irrgang, J. (2016). Patient-specific and surgery-specific factors that affect return to sport after ACL reconstruction. International journal of sports physical therapy, 11(2), 264.
- Abe, S., Kurosaka, M., Iguchi, T., Yoshiya, S., & Hirohata, K. (1993). Light and electron microscopic study of remodeling and maturation process in autogenous graft for anterior cruciate ligament reconstruction. Arthroscopy: The Journal of Arthroscopic & Related Surgery, 9(4), 394-405.
- Claes, S., Verdonk, P., Forsyth, R., & Bellemans, J. (2011). The “ligamentization” process in anterior cruciate ligament reconstruction: what happens to the human graft? A systematic review of the literature. The American journal of sports medicine, 39(11), 2476-2483.
- Falconiero, R. P., DiStefano, V. J., & Cook, T. M. (1998). Revascularization and ligamentization of autogenous anterior cruciate ligament grafts in humans. Arthroscopy: The Journal of Arthroscopic & Related Surgery, 14(2), 197-205.
- Rougraff, B. T., & Shelbourne, K. D. (1999). Early histologic appearance of human patellar tendon autografts used for anterior cruciate ligament reconstruction. Knee Surgery, Sports Traumatology, Arthroscopy, 7(1), 9-14.
- Sánchez, M., Anitua, E., Azofra, J., Prado, R., Muruzabal, F., & Andia, I. (2010). Ligamentization of tendon grafts treated with an endogenous preparation rich in growth factors: gross morphology and histology. Arthroscopy: The Journal of Arthroscopic & Related Surgery, 26(4), 470-480.
- Rabuck, S. J., Baraga, M. G., & Fu, F. H. (2013). Anterior cruciate ligament healing and advances in imaging. Clinics in sports medicine, 32(1), 13-20.
- Muller, B., Bowman, K. F., & Bedi, A. (2013). ACL graft healing and biologics. Clinics in sports medicine, 32(1), 93-109.
- Rabuck, S. J., Baraga, M. G., & Fu, F. H. (2013). Anterior cruciate ligament healing and advances in imaging. Clinics in sports medicine, 32(1), 13-20.
- Amiel, D., Kleiner, J. B., Roux, R. D., Harwood, F. L., & Akeson, W. H. (1986). The phenomenon of “ligamentization”: anterior cruciate ligament reconstruction with autogenous patellar tendon. Journal of orthopaedic research, 4(2), 162-172.
- Joreitz, R., Lynch, A., Rabuck, S., Lynch, B., Davin, S., & Irrgang, J. (2016). Patient-specific and surgery-specific factors that affect return to sport after ACL reconstruction. International journal of sports physical therapy, 11(2), 264.
- Rabuck, S. J., Baraga, M. G., & Fu, F. H. (2013). Anterior cruciate ligament healing and advances in imaging. Clinics in sports medicine, 32(1), 13-20.
- Sánchez, M., Anitua, E., Azofra, J., Prado, R., Muruzabal, F., & Andia, I. (2010). Ligamentization of tendon grafts treated with an endogenous preparation rich in growth factors: gross morphology and histology. Arthroscopy: The Journal of Arthroscopic & Related Surgery, 26(4), 470-480.
- Abe, S., Kurosaka, M., Iguchi, T., Yoshiya, S., & Hirohata, K. (1993). Light and electron microscopic study of remodeling and maturation process in autogenous graft for anterior cruciate ligament reconstruction. Arthroscopy: The Journal of Arthroscopic & Related Surgery, 9(4), 394-405.
- Prodromos, C., Joyce, B., & Shi, K. (2007). A meta-analysis of stability of autografts compared to allografts after anterior cruciate ligament reconstruction. Knee Surgery, Sports Traumatology, Arthroscopy, 15(7), 851-856.
- Rabuck, S. J., Baraga, M. G., & Fu, F. H. (2013). Anterior cruciate ligament healing and advances in imaging. Clinics in sports medicine, 32(1), 13-20.
- Fleming, B. C., Vajapeyam, S., Connolly, S. A., Magarian, E. M., & Murray, M. M. (2011). The use of magnetic resonance imaging to predict ACL graft structural properties. Journal of biomechanics, 44(16), 2843-2846.
- Biercevicz, A. M., Miranda, D. L., Machan, J. T., Murray, M. M., & Fleming, B. C. (2013). In situ, noninvasive, T2*-weighted MRI-derived parameters predict ex vivo structural properties of an anterior cruciate ligament reconstruction or bioenhanced primary repair in a porcine model. The American journal of sports medicine, 41(3), 560-566.
- Myer, G. D., Martin Jr, L., Ford, K. R., Paterno, M. V., Schmitt, L. C., Heidt Jr, R. S., … & Hewett, T. E. (2012). No association of time from surgery with functional deficits in athletes after anterior cruciate ligament reconstruction: evidence for objective return-to-sport criteria. The American journal of sports medicine, 40(10), 2256-2263.
- Adams, D., Logerstedt, D., Hunter-Giordano, A., Axe, M. J., & Snyder-Mackler, L. (2012). Current concepts for anterior cruciate ligament reconstruction: a criterion-based rehabilitation progression. Journal of Orthopaedic & Sports Physical Therapy, 42(7), 601-614.
- Zhu, W., Wang, D., Han, Y., Zhang, N., & Zeng, Y. (2013). Anterior cruciate ligament (ACL) autograft reconstruction with hamstring tendons: clinical research among three rehabilitation procedures. European Journal of Orthopaedic Surgery & Traumatology, 23(8), 939-943.
- Kruse, L. M., Gray, B., & Wright, R. W. (2012). Rehabilitation after anterior cruciate ligament reconstruction: a systematic review. The Journal of bone and joint surgery. American volume, 94(19), 1737.
- Kruse, L. M., Gray, B., & Wright, R. W. (2012). Rehabilitation after anterior cruciate ligament reconstruction: a systematic review. The Journal of bone and joint surgery. American volume, 94(19), 1737.
- Adams, D., Logerstedt, D., Hunter-Giordano, A., Axe, M. J., & Snyder-Mackler, L. (2012). Current concepts for anterior cruciate ligament reconstruction: a criterion-based rehabilitation progression. Journal of Orthopaedic & Sports Physical Therapy, 42(7), 601-614.
- Kruse, L. M., Gray, B., & Wright, R. W. (2012). Rehabilitation after anterior cruciate ligament reconstruction: a systematic review. The Journal of bone and joint surgery. American volume, 94(19), 1737.
- Shelbourne, K. D., Freeman, H., & Gray, T. (2012). Osteoarthritis after anterior cruciate ligament reconstruction: the importance of regaining and maintaining full range of motion. Sports health, 4(1), 79-85.
- Lepley, L. K., & Palmieri-Smith, R. M. (2013). Effect of eccentric strengthening after anterior cruciate ligament reconstruction on quadriceps strength. Journal of sport rehabilitation, 22(2), 150-156.
- Kim, K. M., Croy, T., Hertel, J., & Saliba, S. (2010). Effects of neuromuscular electrical stimulation after anterior cruciate ligament reconstruction on quadriceps strength, function, and patient-oriented outcomes: a systematic review. Journal of Orthopaedic & Sports Physical Therapy, 40(7), 383-391.
- Papandreou, M., Billis, E., Papathanasiou, G., Spyropoulos, P., & Papaioannou, N. (2013). Cross-exercise on quadriceps deficit after ACL reconstruction. J Knee Surg, 26(1), 51-8.
- Lobb, R., Tumilty, S., & Claydon, L. S. (2012). A review of systematic reviews on anterior cruciate ligament reconstruction rehabilitation. Physical Therapy in Sport, 13(4), 270-278.
- Kruse, L. M., Gray, B., & Wright, R. W. (2012). Rehabilitation after anterior cruciate ligament reconstruction: a systematic review. The Journal of bone and joint surgery. American volume, 94(19), 1737.
- Nyland, J., Mauser, N., & Caborn, D. N. M. (2013). Sports involvement following ACL reconstruction is related to lower extremity neuromuscular adaptations, subjective knee function and health locus of control. Knee Surgery, Sports Traumatology, Arthroscopy, 21(9), 2019-2028.
- Angelozzi, M., Madama, M., Corsica, C., Calvisi, V., Properzi, G., McCaw, S. T., & Cacchio, A. (2012). Rate of force development as an adjunctive outcome measure for return-to-sport decisions after anterior cruciate ligament reconstruction. Journal of Orthopaedic & Sports Physical Therapy, 42(9), 772-780.
- Aagaard, P., Simonsen, E. B., Andersen, J. L., Magnusson, P., & Dyhre-Poulsen, P. (2002). Increased rate of force development and neural drive of human skeletal muscle following resistance training. Journal of applied physiology, 93(4), 1318-1326.
- Kraemer, W. J., Adams, K., Cafarelli, E., Dudley, G. A., Dooly, C., Feigenbaum, M. S., … & Newton, R. U. (2002). American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Medicine and science in sports and exercise, 34(2), 364-380.
- Angelozzi, M., Madama, M., Corsica, C., Calvisi, V., Properzi, G., McCaw, S. T., & Cacchio, A. (2012). Rate of force development as an adjunctive outcome measure for return-to-sport decisions after anterior cruciate ligament reconstruction. Journal of Orthopaedic & Sports Physical Therapy, 42(9), 772-780.
- MacDonald, P. B., Hedden, D., Pacin, O., & Huebert, D. (1995). Effects of an accelerated rehabilitation program after anterior cruciate ligament reconstruction with combined semitendinosus-gracilis autograft and a ligament augmentation device. The American Journal of sports medicine, 23(5), 588-592.
- Maffiuletti, N. A., Bizzini, M., Widler, K., & Munzinger, U. (2010). Asymmetry in quadriceps rate of force development as a functional outcome measure in TKA. Clinical Orthopaedics and Related Research, 468(1), 191-198.
- Angelozzi, M., Madama, M., Corsica, C., Calvisi, V., Properzi, G., McCaw, S. T., & Cacchio, A. (2012). Rate of force development as an adjunctive outcome measure for return-to-sport decisions after anterior cruciate ligament reconstruction. Journal of Orthopaedic & Sports Physical Therapy, 42(9), 772-780.
- Ardern, C. L., Webster, K. E., Taylor, N. F., & Feller, J. A. (2011). Return to sport following anterior cruciate ligament reconstruction surgery: a systematic review and meta-analysis of the state of play. Br J Sports Med, 45(7), 596-606.
- Ardern, C. L., Webster, K. E., Taylor, N. F., & Feller, J. A. (2011). Return to sport following anterior cruciate ligament reconstruction surgery: a systematic review and meta-analysis of the state of play. Br J Sports Med, 45(7), 596-606.
- Stone, M. H., Sands, W. A., Carlock, J. O. N., Callan, S. A. M., Dickie, D. E. S., Daigle, K., … & Hartman, M. (2004). The importance of isometric maximum strength and peak rate-of-force development in sprint cycling. The Journal of Strength & Conditioning Research, 18(4), 878-884.
- Aagaard, P., Simonsen, E. B., Andersen, J. L., Magnusson, P., & Dyhre-Poulsen, P. (2002). Increased rate of force development and neural drive of human skeletal muscle following resistance training. Journal of applied physiology, 93(4), 1318-1326.
- Beard, D. J., Kyberd, P. J., Fergusson, C. M., & Dodd, C. A. (1993). Proprioception after rupture of the anterior cruciate ligament. An objective indication of the need for surgery?. The Journal of bone and joint surgery. British volume, 75(2), 311-315.
- Wojtys, E. M., & Huston, L. J. (1994). Neuromuscular performance in normal and anterior cruciate ligament-deficient lower extremities. The American journal of sports medicine, 22(1), 89-104.
- Swanik, C. B., Lephart, S. M., Giraldo, J. L., DeMont, R. G., & Fu, F. H. (1999). Reactive muscle firing of anterior cruciate ligament-injured females during functional activities. Journal of athletic training, 34(2), 121.
- Sheppard, J. M., Cormack, S., Taylor, K. L., McGuigan, M. R., & Newton, R. U. (2008). Assessing the force-velocity characteristics of the leg extensors in well-trained athletes: the incremental load power profile. The Journal of Strength & Conditioning Research, 22(4), 1320-1326.
- Kraemer, W. J., Adams, K., Cafarelli, E., Dudley, G. A., Dooly, C., Feigenbaum, M. S., … & Newton, R. U. (2002). American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Medicine and science in sports and exercise, 34(2), 364-380.
- Angelozzi, M., Madama, M., Corsica, C., Calvisi, V., Properzi, G., McCaw, S. T., & Cacchio, A. (2012). Rate of force development as an adjunctive outcome measure for return-to-sport decisions after anterior cruciate ligament reconstruction. Journal of Orthopaedic & Sports Physical Therapy, 42(9), 772-780.
- Kamath, G. V., Redfern, J. C., Greis, P. E., & Burks, R. T. (2011). Revision anterior cruciate ligament reconstruction. The American journal of sports medicine, 39(1), 199-217.
- Mizner, R. L., Kawaguchi, J. K., & Chmielewski, T. L. (2008). Muscle strength in the lower extremity does not predict postinstruction improvements in the landing patterns of female athletes. Journal of Orthopaedic & Sports Physical Therapy, 38(6), 353-361.
- Lind, M., Menhert, F., & Pedersen, A. B. (2012). Incidence and outcome after revision anterior cruciate ligament reconstruction: results from the Danish registry for knee ligament reconstructions. The American journal of sports medicine, 40(7), 1551-1557.
- Wasserstein, D., Khoshbin, A., Dwyer, T., Chahal, J., Gandhi, R., Mahomed, N., & Ogilvie-Harris, D. (2013). Risk factors for recurrent anterior cruciate ligament reconstruction: a population study in Ontario, Canada, with 5-year follow-up. The American journal of sports medicine, 41(9), 2099-2107.
- Shelbourne, K. D., Gray, T., & Haro, M. (2009). Incidence of subsequent injury to either knee within 5 years after anterior cruciate ligament reconstruction with patellar tendon autograft. The American journal of sports medicine, 37(2), 246-251.
- Hui, C., Salmon, L. J., Kok, A., Maeno, S., Linklater, J., & Pinczewski, L. A. (2011). Fifteen-year outcome of endoscopic anterior cruciate ligament reconstruction with patellar tendon autograft for “isolated” anterior cruciate ligament tear. The American journal of sports medicine, 39(1), 89-98.
- Pinczewski, L. A., Lyman, J., Salmon, L. J., Russell, V. J., Roe, J., & Linklater, J. (2007). A 10-year comparison of anterior cruciate ligament reconstructions with hamstring tendon and patellar tendon autograft: a controlled, prospective trial. The American journal of sports medicine, 35(4), 564-574.
- Wright, R. W., Magnussen, R. A., Dunn, W. R., & Spindler, K. P. (2011). Ipsilateral graft and contralateral ACL rupture at five years or more following ACL reconstruction: a systematic review. The Journal of Bone and Joint Surgery. American volume., 93(12), 1159.
- Webster, K. E., Feller, J. A., Leigh, W. B., & Richmond, A. K. (2014). Younger patients are at increased risk for graft rupture and contralateral injury after anterior cruciate ligament reconstruction. The American journal of sports medicine, 42(3), 641-647.
- Kaeding, C. C., Pedroza, A. D., Reinke, E. K., Huston, L. J., Moon Consortium, & Spindler, K. P. (2015). Risk factors and predictors of subsequent ACL injury in either knee after ACL reconstruction: prospective analysis of 2488 primary ACL reconstructions from the MOON cohort. The American journal of sports medicine, 43(7), 1583-1590.
- Maletis, G. B., Chen, J., Inacio, M. C., & Funahashi, T. T. (2016). Age-related risk factors for revision anterior cruciate ligament reconstruction: a cohort study of 21,304 patients from the Kaiser Permanente Anterior Cruciate Ligament Registry. The American journal of sports medicine, 44(2), 331-336.
- Gifstad, T., Foss, O. A., Engebretsen, L., Lind, M., Forssblad, M., Albrektsen, G., & Drogset, J. O. (2014). Lower risk of revision with patellar tendon autografts compared with hamstring autografts: a registry study based on 45,998 primary ACL reconstructions in Scandinavia. The American journal of sports medicine, 42(10), 2319-2328.
- Ahldén, M., Samuelsson, K., Sernert, N., Forssblad, M., Karlsson, J., & Kartus, J. (2012). The Swedish National Anterior Cruciate Ligament Register: a report on baseline variables and outcomes of surgery for almost 18,000 patients. The American journal of sports medicine, 40(10), 2230-2235.
- Andernord, D., Desai, N., Björnsson, H., Ylander, M., Karlsson, J., & Samuelsson, K. (2015). Patient predictors of early revision surgery after anterior cruciate ligament reconstruction: a cohort study of 16,930 patients with 2-year follow-up. The American journal of sports medicine, 43(1), 121-127.
- Lind, M., Menhert, F., & Pedersen, A. B. (2012). Incidence and outcome after revision anterior cruciate ligament reconstruction: results from the Danish registry for knee ligament reconstructions. The American journal of sports medicine, 40(7), 1551-1557.
- Persson, A., Fjeldsgaard, K., Gjertsen, J. E., Kjellsen, A. B., Engebretsen, L., Hole, R. M., & Fevang, J. M. (2014). Increased risk of revision with hamstring tendon grafts compared with patellar tendon grafts after anterior cruciate ligament reconstruction: a study of 12,643 patients from the Norwegian Cruciate Ligament Registry, 2004-2012. The American journal of sports medicine, 42(2), 285-291.
- Dekker, T. J., Godin, J. A., Dale, K. M., Garrett, W. E., Taylor, D. C., & Riboh, J. C. (2017). Return to sport after pediatric anterior cruciate ligament reconstruction and its effect on subsequent anterior cruciate ligament injury. JBJS, 99(11), 897-904.
- Paterno, M. V., Rauh, M. J., Schmitt, L. C., Ford, K. R., & Hewett, T. E. (2012). Incidence of contralateral and ipsilateral anterior cruciate ligament (ACL) injury after primary ACL reconstruction and return to sport. Clinical journal of sport medicine: official journal of the Canadian Academy of Sport Medicine, 22(2), 116.
- Joreitz, R., Lynch, A., Rabuck, S., Lynch, B., Davin, S., & Irrgang, J. (2016). Patient-specific and surgery-specific factors that affect return to sport after ACL reconstruction. International journal of sports physical therapy, 11(2), 264.
- Chmielewski, T. L. (2011). Asymmetrical lower extremity loading after ACL reconstruction: more than meets the eye. J Orthop Sports Phys Ther; 41(6):374-376
- Myer, G. D., Paterno, M. V., Ford, K. R., Quatman, C. E., & Hewett, T. E. (2006). Rehabilitation after anterior cruciate ligament reconstruction: criteria-based progression through the return-to-sport phase. Journal of Orthopaedic & Sports Physical Therapy, 36(6), 385-402.
- Myer, G. D., Schmitt, L. C., Brent, J. L., Ford, K. R., Barber Foss, K. D., Scherer, B. J., … & Hewett, T. E. (2011). Utilization of modified NFL combine testing to identify functional deficits in athletes following ACL reconstruction. Journal of orthopaedic & sports physical therapy, 41(6), 377-387.
- Hewett, T. E., Di Stasi, S. L., & Myer, G. D. (2013). Current concepts for injury prevention in athletes after anterior cruciate ligament reconstruction. The American journal of sports medicine, 41(1), 216-224.
- Kiefer, A. W., Ford, K. R., Paterno, M. V., Schmitt, L. C., Myer, G. D., Riley, M. A., … & Hewett, T. E. (2013). Inter-segmental postural coordination measures differentiate athletes with ACL reconstruction from uninjured athletes. Gait & posture, 37(2), 149-153.
- Aagaard, P., Simonsen, E. B., Magnusson, S. P., Larsson, B., & Dyhre-Poulsen, P. (1998). A new concept for isokinetic hamstring: quadriceps muscle strength ratio. The American journal of sports medicine, 26(2), 231-237.
- Baltaci, G., Yilmaz, G., & Atay, A. O. (2012). The outcomes of anterior cruciate ligament reconstructed and rehabilitated knees versus healthy knees: a functional comparison. Acta Orthop Traumatol Turc, 46(3), 186-195.
- Feller, J., & Webster, K. E. (2013). Return to sport following anterior cruciate ligament reconstruction. International orthopaedics, 37(2), 285-290.
- Kocher, M. S., Sterett, W. I., Zurakowski, D., & Steadman, J. R. (2003). Effect of Functional Bracing on Subsequent Knee Injury in ACL-Deficient Professional Skiers. The journal of knee surgery, 16, 87-92.
- DiSanti, J., Lisee, C., Erickson, K., Bell, D., Shingles, M., & Kuenze, C. (2018). Perceptions of Rehabilitation and Return to Sport Among High School Athletes With ACL Reconstruction: A Qualitative Research Study. Journal of Orthopaedic & Sports Physical Therapy, (0), 1-31.
- Ardern, C. L., Taylor, N. F., Feller, J. A., & Webster, K. E. (2014). Fifty-five per cent return to competitive sport following anterior cruciate ligament reconstruction surgery: an updated systematic review and meta-analysis including aspects of physical functioning and contextual factors. Br J Sports Med, 48(21), 1543-1552.
- Flanigan, D. C., Everhart, J. S., Pedroza, A., Smith, T., & Kaeding, C. C. (2013). Fear of reinjury (kinesiophobia) and persistent knee symptoms are common factors for lack of return to sport after anterior cruciate ligament reconstruction. Arthroscopy: The Journal of Arthroscopic & Related Surgery, 29(8), 1322-1329.
- Kvist, J., Ek, A., Sporrstedt, K., & Good, L. (2005). Fear of re-injury: a hindrance for returning to sports after anterior cruciate ligament reconstruction. Knee surgery, sports traumatology, arthroscopy, 13(5), 393-397.
- Lentz, T. A., Zeppieri Jr, G., Tillman, S. M., Indelicato, P. A., Moser, M. W., George, S. Z., & Chmielewski, T. L. (2012). Return to preinjury sports participation following anterior cruciate ligament reconstruction: contributions of demographic, knee impairment, and self-report measures. Journal of orthopaedic & sports physical therapy, 42(11), 893-901.
- Feller, J., & Webster, K. E. (2013). Return to sport following anterior cruciate ligament reconstruction. International orthopaedics, 37(2), 285-290.
- Ardern, C. L., Taylor, N. F., Feller, J. A., & Webster, K. E. (2012). Fear of re-injury in people who have returned to sport following anterior cruciate ligament reconstruction surgery. Journal of science and medicine in sport, 15(6), 488-495.
- Ardern, C. L., Taylor, N. F., Feller, J. A., Whitehead, T. S., & Webster, K. E. (2013). Psychological responses matter in returning to preinjury level of sport after anterior cruciate ligament reconstruction surgery. The American journal of sports medicine, 41(7), 1549-1558.
- DiSanti, J., Lisee, C., Erickson, K., Bell, D., Shingles, M., & Kuenze, C. (2018). Perceptions of Rehabilitation and Return to Sport Among High School Athletes With ACL Reconstruction: A Qualitative Research Study. Journal of Orthopaedic & Sports Physical Therapy, (0), 1-31.
- Salmon, L. J., Heath, E., Akrawi, H., Roe, J. P., Linklater, J., & Pinczewski, L. A. (2018). 20-Year outcomes of anterior cruciate ligament reconstruction with hamstring tendon autograft: the catastrophic effect of age and posterior tibial slope. The American journal of sports medicine, 46(3), 531-543.
- Salmon, L. J., Heath, E., Akrawi, H., Roe, J. P., Linklater, J., & Pinczewski, L. A. (2018). 20-Year outcomes of anterior cruciate ligament reconstruction with hamstring tendon autograft: the catastrophic effect of age and posterior tibial slope. The American journal of sports medicine, 46(3), 531-543.
- DiSanti, J., Lisee, C., Erickson, K., Bell, D., Shingles, M., & Kuenze, C. (2018). Perceptions of Rehabilitation and Return to Sport Among High School Athletes With ACL Reconstruction: A Qualitative Research Study. Journal of Orthopaedic & Sports Physical Therapy, (0), 1-31.
- DiSanti, J., Lisee, C., Erickson, K., Bell, D., Shingles, M., & Kuenze, C. (2018). Perceptions of Rehabilitation and Return to Sport Among High School Athletes With ACL Reconstruction: A Qualitative Research Study. Journal of Orthopaedic & Sports Physical Therapy, (0), 1-31.
