Introduction
Shoulder pain represents the 3rd most common musculoskeletal complaint.1 2 Rotator Cuff ( RTC ) Injuries run the spectrum from mild strains to full rupture of multiple muscles. Often the entire range of injury or dysfunction will be referred to ‘Rotator cuff diseases’. The array of impairments, pain and limitations associated with this process increases with age, but can, and do, afflict the young and healthy as well.
As a whole, Shoulder disorders are quite common. Up to 50% of the general population will experience at least one episode of shoulder pain annually. 54% of these ‘disorders’ will continue to cause Symptoms 3 years down the road. 3
So, problems in this region are pervasive and do not necessarily ‘go away on their own.’
Damage to the RTC can occur as a result of degenerative changes (and is, to some degree, part of the natural aging process), repetitive micro traumas, and severe traumatic injuries. 4
Why is the RTC ‘vulnerable’ to injury?
Our RTC is particularly prone to injury for a number of reasons:
1) The wide mobility of the shoulder joint and the high demands placed on it in particular with athletics and manual labor, create an environment of repetitive strain.
2) The anatomy of the shoulder creates multiple weak spots. This includes ‘impingement points’ where the muscles and tendons rub over, or are impinged by, rough boney protuberances creating focal areas of mechanical stress.
* See below for discussion of ‘Impingement Syndrome’.
3) Other particularly susceptible portions of the RTC include tendons with poor blood supply, and thus limited healing potential, making it more prone to repetitive stress breakdown. 5 6 7
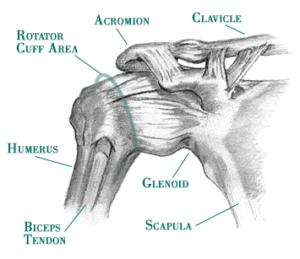
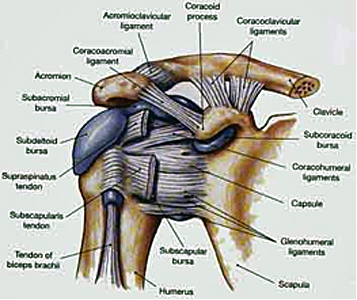
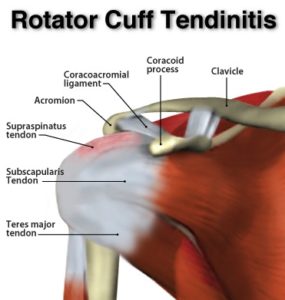
Anatomy
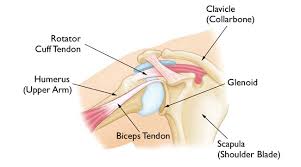
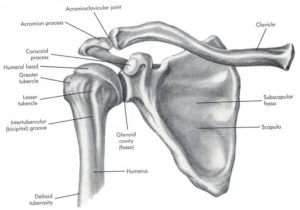
– Joint – The ‘Shoulder Girdle’ is made up by the arm bone (the humerus), the Shoulder Blade (the scapula) and the clavicle (the collar bone). The connection between your clavicle and scapula is the only boney attachment between your arm and your trunk; the arm is primarily held on to the body with muscles.
The ‘Shoulder Joint’ is properly named the ‘Glenohumeral Joint’. It is the ball-and-socket attachment of head of the humerus (the ‘ball’) and the glenoid (‘socket’) of the scapula (shoulder blade).
The rotator cuff muscle group act together to ‘stabilize’ and center the ball in the socket, preventing ‘sloppy’ movement in the joint and to prevent the humeral head (the ‘ball’) from riding high in the ‘socket’ (the glenoid) when the arm is elevated over head. This is important to prevent impingement of the structures on the top of the joint (described below).
Additionally, each muscle, in isolation, contributes a unique movement:
Subscapularis – Provides medial (inward) rotation (think ‘forehand’ swing)
Supraspinatus – Helps the Deltoid with ‘Abduction’ (raising the arm straight out to the side).
Infraspinatus – Provides lateral (outward) rotation (think ‘backhand’ swing)
Teres Minor – Provides lateral (outward) rotation (think ‘backhand’ swing) and also helps with extension.
There is considerable ‘overlap’ in function from one muscle to the other. For this reason, even in the face of a massive Supraspinatus Tear (for example) an intact and healthy Subscapularis, working in conjunction with the Infraspinatus and Teres, may be able to compensate for the lost stability. 8
Long Head of Biceps – The tendon of the “long head” of the biceps is intimately associated with the RTC. Its exact mechanism of action is entirely known (and is the source of some debate). But, it appears as though it may assist with humeral head stability by resisting superior (upward) translation and anterior (forward) ‘shearing’ during rotational movements. 9 However, Biceps Tenotomy surgeries (where this tendon is completely severed and removed) seems to result in little functional loss. Therefor it’s contribution to ‘stability’ is likely small. Other relevant anatomy include the Bursae sacs that surround the joint and separate various boney structures and tendons.
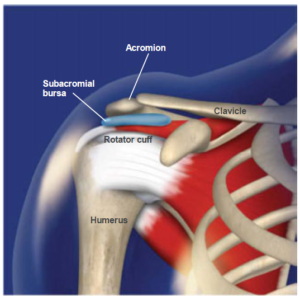
Impingement
Impingement Syndrome can refer to various mechanical causes of painful ‘pinching’, or ‘rubbing’ of structures surrounding the shoulder joint. Subacromial pain is the most common form of shoulder pain, accounting for unto 70% of all cases.10 11 12 There are many causes of ‘Impingement-type’ symptoms and pain. Traditionally, the term impingement refers to compression of the RTC and/or subacromial bursa by any of the following: the AC joint, anterior acromion, coracoacromial arch, and/or the coracoacromial ligament. In reality, this ‘Syndrome’ is likely not differentiable from various RTC tendonopathies and bursitis. In fact, a proposed classification syndrome lists “degeneration and partial thickness rotator cuff tears or abnormalities in the subacromial bursa on imaging” as a key finding in making the diagnosis of ’Subacromial Pain Syndrome’.13
Shoulder Impingement is very common – Estimates suggest that 1 in 3 people will experience painful shoulder impingement, and this number goes up in the athletic population.
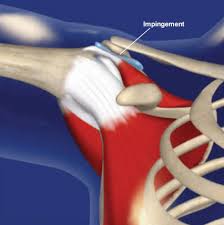
The most common causes of Impingement Syndrome include:
Functional Causes:
- Hypermobility (laxity) of the Shoulder (glenohumeral) joint – This allows the humeral head (the ‘ball’) to slide around in the socket excessively, potentially compressing against adjacent structures.
- Weakness of the RTC – This allows the humeral head to drift ‘upward’ in the socket when you raise your arms up in the air. Typically, the RTC acts to keep the ball centered in the socket while the arm moves in space.
- Postural Dysfunction – Habitual posturing with the upper back flexed (or ‘slouched’), the shoulders rotated forward and the head ‘protruded’, are all very common contributing factors to Impingement related injuries.
* These ‘functional’ causes of Impingement are not due to a structural deformity or anomaly. This is referred to as secondary impingement; in these cases a surgical ‘decompression’ is not indicated.
Structural Causes:
- Acromion ‘Spurs’ (AKA a ‘hooked’ acromion) – basically, the end of the acromion can thicken and develop bone spurring that give it a ‘hooked’ appearance. This overhang can impinge against the top of the humeral head causing irritation and/or damage to the soft tissues b/w the two structures (i.e. your supraspinatus tendon and subacromial bursa)
- Acromial Clavicular (AC) Joint Degeneration – Spurring of the distal clavicle in the presence of degenerative changes of the AC joint can lead to a similar mechanism of impingement. This may occur as the result of repetitive strain and/or trauma
(Click here for more detail information on AC joint Injuries)
- Sub-deltoid and Sub-acromial Bursitis – A bursa sac is a fluid filled pouch positions at various places throughout the body to separate moving structures in order to reduce friction and prevent rubbing wear and tear. When the bursae are not irritated or inflamed, structures glide over them in a smooth and painless way. When the bursae become inflamed however, due to injury or repetitive stress, any movement that compresses the sac will cause pain. In fact, the bursa sac is more highly innervated by pain receptors than most of the surrounding tissues (including the RTC tendons themselves). 14 15
Bursitis in the shoulder is more common in younger and middle-aged patients and will typically be associated with pain when elevating the arm to the side, overhead, and/or sleeping on the effected side.
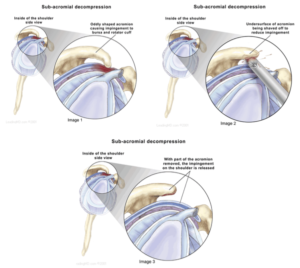
* These structural anomalies are commonly referred to as Primary Impingement. ‘Acromioplasty’and/or Decompression’ surgery may be indicated.
‘Decompression’ refers to the removal of tissue between the arm bone and the acromion – typically including the bursa sac.
‘Acromioplasty’ refers to the shaving down of the end of the acromion.
Regardless of the cause – there is very strong evidence for the effectiveness of Physical Therapy for Shoulder Impingement.
Multiple studies have looked at patients with Impingement Syndrome and compared their outcomes with surgical intervention, to Rehabilitation (with, or without corticosteroid injections and/or oral anti-inflammatories). 16 17 18
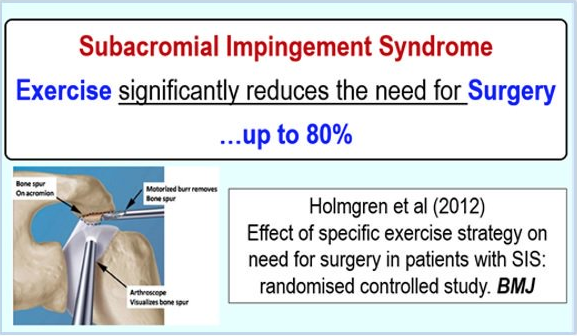
All of the studies referenced above failed to show a difference in outcomes with surgery in general. One particularly well-done study looked at 140 patients diagnosed with impingement syndrome. They were broken into 2 groups – 1) Treated with a supervised exercise program, and 2) Given a surgical ‘acromioplasty’ and then followed up with a supervised exercise program similar to the first group. Patients were followed for 5 years! The paper reports no added benefit from the surgery. The authors conclude,
“structured exercise treatment seems to be the treatment of choice for shoulder impingement syndrome” 19
A conclusion supported again 20 and again. 21
Recently a Clinical Practice Guideline was published on this topic in the British Medical Journal.22 They found, in short, evidence to suggest that “decompression” surgery provides “no benefit over placebo surgery”. And, surgical interventions for SAD carry significant risks of post-surgical adhesive capsulitis (i.e. ‘Frozen Shoulder’). A panel of experts “concluded that almost all informed patients would choose to avoid surgery because there is no benefit but there are harms and it is burdensome”.23
Importantly, some patients do eventually benefit from surgical intervention. The tricky part is identifying who is a ‘candidate’ when the majority of patients will do just as well with a Physical Therapy only approach. For the most part, patients who have not responded to ~3-4 months of Physical Therapy are considered candidates for surgery.
When surgery is indicated, there is some controversy as to the ‘best’ approach. Recently, the drive to ‘remodel’ or shave down the acromion has been called into question.
Multiple surgeons and researchers have suggested that the ‘decompression’ of the subacromial space by removal of swollen irritated tissue (such as the bursa) provide benefit and an ‘Acromioplasty’ (the shaving down of the bone and/or removal of the coracoacromial ligament) may be ill advised. It appears that decompression alone is an appropriate treatment. 24 This contention was supported by a Prospective randomized clinical trial 25 and a Systematic Review concluding that “bursectomy alone provides similar outcomes to bursectomy with acromioplasty” 26
A 2011 Review in the Journal of Bone and Joint Surgery even calls into question (rightfully in my opinion) the usefulness of the term ‘Impingement Syndrome’ it’s self. The authors point out that:
1) The clinical tests we used to ‘diagnose’ impingement syndrome are not really specific to this ‘disease’.
2) Wear patterns in RTC tendons often do not ‘line up’ with the site of impingement against the acromion, and are therefore not likely to be due to mechanical rubbing or compression.
3) RTC contact with the ‘coracoacromial’ arch is a normal occurrence and should not be assumed to occur only in pathological cases.
4) ‘Spurs’ on the end of the acromion rarely extend beyond the space occupied by the coracoacromial ligament.
5) The shape of the acromion and/or the coracoacromial ligament does not need to be altered to successfully treat patients with presentations consistent with the current use of the term ‘Impingement Syndrome’.
The article points out that Corticosteroid Injections and Physical Therapy have been shown to be effective for treating ‘Impingement Syndrome’ and “studies have failed to show the superiority of acromioplasty over (these) alternative treatments”. 27
The author’s final discussion points (emphasis added):
This investigation did not reveal evidential support for the ongoing use of the term impingement syndrome or for the use of this diagnosis as an indication for acromioplasty. The evidence does not support the view that such a syndrome can be differentiated from conditions… such as rotator cuff tendinosis, partial-thickness tears, and fullthickness tears. The evidence does not support the view that rotator cuff abnormality is, in most cases, caused by rubbing on the coracoacromial arch. Rather, the evidence indicates that the rotator cuff articulates with the coracoacromial arch in the movement of normal shoulders… contact between the coracoacromial arch and the rotator cuff normally stabilizes the humerus… suggesting that the coracoacromial arch has an important duty and should not be thoughtlessly divided at any operation.”

It appears that ‘Impingement Syndrome’ may be a description of symptoms rather than a true ‘stand-alone’ diagnosis.
In other words, the impingement may result from irritated, sensitive tissues being exposed to normal mechanical strain, rather than cause the irritation it’s self.
In summary, irrespective of the ‘cause-and-effect’, both the inflamed tissue AND the dysfunctional mechanics need to be addressed in treatment; and, regardless of the presence or absence of structural deformity surrounding the shoulder (with very few exceptions)
All patients with “Shoulder Impingement” should be treated with Physical Therapy before considering any surgical intervention.
Also, Glucocorticoid injections and NSAIDs “may provide moderate to small short term benefits on shoulder pain compared with placebo” 28 29 30
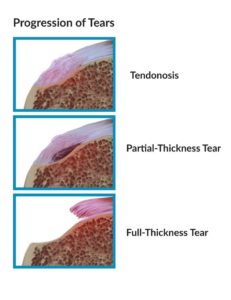
Tendonitis / Tendinosis
Rotator Cuff Tendonitis refers to an acute (or subacute) inflammation of the RTC tendons, most typically as they course over the top of the humeral head, near their attachment point on the ‘tip’ of the shoulder. Rotator Cuff Tendonosis refers to chronic degeneration of the RTC tendons. This is often a gradual ‘wear and tear’ dysfunction associated with RTC and shoulder girdle weakness / deconditioning, poor postural habits, and/or sports or work related overhead use. Additionally, certain ‘systemic’ issues are risk factors for developing RTC Tendonosis – These include Smoking, Diabetes, and being overweight.
While the onset of pain can be associated with a sudden onset, or an injury / event, more typically the onset of Tendonitis and Tendonosis pain is gradual. It seems to come out of nowhere and build over a period of time. Patients often report a dull, vague achiness in the area of the shoulder joint occasionally radiating into the upper arm. Often the pain increases with certain movements – the particular direction of provocation depends on the muscle(s)/tendon(s) involved. Common difficulties include reaching across the body (think, putting on deodorant), overhead or behind your body (think, tucking in a shirt).
Common clinical tests can usually differentiate Tendonopathy from a RTC tear. However, it may be useful to think of ‘RTC disease’ as a continuum with Tendonitis and Impingement-type problems on one end of the spectrum… and full Tearing and failure of the RTC on the other end of the spectrum.
Unfortunately – Many people do not seek the help of a Physical Therapist when they are towards the top of the spectrum. This is a mistake!
A comprehensive Physical Therapy approach is effective to return proper mobility and reduce pain in cases of shoulder tendonopathy. 31
Perhaps more importantly, it may slow or halt the progression towards more advanced disease and/or tear.
Such an intervention should focus on returning proper movement patterns, balancing postural forces across the shoulder girdle and improving the strength & endurance of both the RTC and the scapular muscles.
* A long term conditioning program must be implemented to prevent a return, or a worsening of symptoms *
Side Note
An overreliance on passive modalities – such as Ultrasound or Low-Level Laser treatments – may be a waste of your time 32
If you’re spending most of your Physical Therapy session lying on a table while a magic wand is waived over your shoulder… or, even worse, blowing up balloons… you may be wasting your time. (Here is an example of why that is!)
Rotator Cuff Tears
A Tear within one or more of the rotator cuff muscles (or tendons) can occur – possibly (but not always) causing pain, weakness and/or compensatory movements/use. Symptoms of rotator cuff tears vary from person to person. Often a patient will complain of a dull, aching pain in the front and side of the shoulder, which can radiate into the upper arm. This pain may be more sharp or severe with an acute tear.
Other common symptoms of a rotator cuff tear include:
- Severe pain at time of injury (if traumatic)
- Pain at night
- Pain with overhead activities
- Positive “painful arc” sign (pain when arm is away from side ~ shoulder level)
- Weakness of the involved muscle(s)
- A feeling of shoulder stiffness. 33
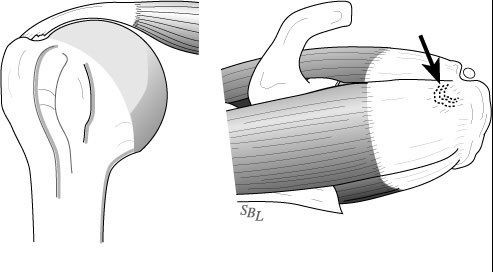
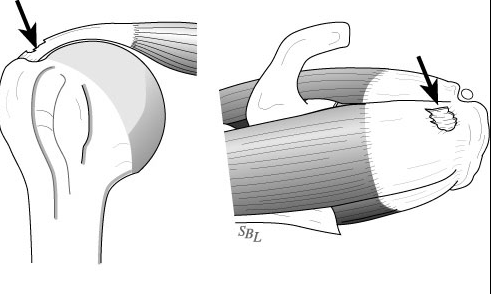
Most commonly, the tear involves the Supraspinatus (the RTC muscle on the top of the shoulder) with, or without adjacent muscles / tendons. These tears can be either ‘Partial Thickness’ or ‘Full Thickness’. This is somewhat self-explanatory – partial thickness tears only involving a portion of the muscle/tendon, but does not extend the full width of the muscle.
These partial thickness tears are either ‘Articular’ surface tears (shown above) or, less commonly ‘Bursal’ side tears (along the outside of the muscle – shown below). Rarely, but more commonly in overhead athletes, the tear can be ‘Interstitial’ – within the center of the muscle it’s self.
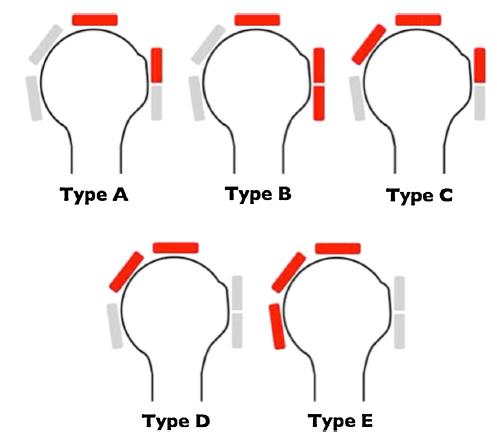
Rotator cuff tears may be reference by ‘Type’. This classification system was proposed by Collin. 34
Type A: supraspinatus & superior subscapularis tears (i.e. a “Superior” Tear)
Type B: supraspinatus and entire subscapularis tears (a “Superior-Anterior” Tear)
Type C: supraspinatus, superior subscapularis & infraspinatus tears
Type D: supraspinatus & infraspinatus tears (a “Superior-Posterior” Tear)
Type E: supraspinatus, infraspinatus & teres minor tear (a “Superior-Posterior” Tear)
More commonly, RTC tears will be referenced by Size (for full-thickness tears):
Small: less than 1 cm (usually involving the supraspinatus)
Medium: 1–3 cm (usually involving the supraspinatus)
Large: 3–5 cm (usually involving the supraspinatus + infraspinatus or Subscapularis)
Massive: greater than 5 cm. (usually involving the supraspinatus + infraspinatus + Subscapularis)
Prevalence
It is important to consider, 4% of asymptomatic patients aged less than 40 years and 54% of patients aged over 60 years, have partial or complete tears of the rotator cuff on a MRI scan. 35 Thus, we must consider ‘degenerative’ tearing of the RTC as, at least to some degree, a part of natural aging.
This tissue breakdown may, or may not become a problem for the individual based on a number of factors – size of the tear, location of the tear, activity level of the patient, sports-specific demands of the athlete, the quality of the surrounding tissues and the ability to ‘compensate’, etc. There is considerable controversy as to how aggressive RTC tears should be treated, and with what intervention. In addition, when a patient is having shoulder pain, and a RTC tear is found on imaging, is it possible that the pain is not related to the tear? That the tear is an ‘incidental finding’? We know there are thousands upon thousands of people walking around with torn cuffs, having no pain at all. There is certainly a chance that someone could experience another source of shoulder pain, only to then find this (pre-existing) tear upon getting an MRI.
Given the complex and multi-factorial nature of these decisions… AND the discrepancy in the literature (conflicting evidence), patients are strongly recommended to consult with a shoulder specialist who can perform an individualized evaluation to determine the best treatment route. Otherwise, one runs the risk of talking in circles as to what the ‘right’ thing to do is.
Otherwise, one runs the risk of talking in circles as to what the ‘right’ thing to do is.
The following is some information to weigh when deciding the best route to go:
Partial tears are suggested to ‘concentrate’ the force to the remaining intact tissue, and thus lead to full rupture of the muscle / cuff. This has long been accepted (and likely holds true, to some degree, for some people). One longitudinal study found that more than half of asymptomatic rotator cuff tears become symptomatic within 3 years and progressed in size during this time period 36 Another study looking into the ‘natural progression’ of RTC disease followed patients with ‘partial thickness’ tears. They found that that 10% of these partial tears healed, 10% reduced in size, 53% advanced and 28% progressed to be a ‘full-thickness’ tear. 37 Multiple studies have suggested that full-thickness tears do not spontaneously heal, and may worsen with time38 39 – not very encouraging, huh?
However, a more recent report in the Journal of Bone and Joint Surgery challenges the belief that a worsening of tears over time is a fore gone conclusion. A report of 24 patients with ‘full-thickness’ tears of the Supraspinatus who ‘opted out’ of surgery were followed for more than 3 years after original diagnosis. They found that, on average, the tear did not increase in size over time. In fact, some appeared to be improving. In two shoulders, the tear was no longer detectable on MRI and in nine shoulders the tear was ‘smaller’ than it had been at the time of the initial diagnosis (in nine patients the tear had not changed, and in six patients the tear had increased in size). 40
Keep in mind, this is ‘improvement’ as evidenced by imaging (i.e. it looks better on the MRI). ‘Improvement’ with regards to the patient experience (i.e. hurts less, moves better, functions better, etc.) is something that we know happens often.
What to Do if Diagnosed with a Rotator Cuff Disease?
Conservative Treatment
In most cases, the recommendation is for an initial period of “conservative Management”. 41 This should include 2-3 months of Physical Therapy at a minimum, with perhaps some level of activity modification during that time. 42 43 44
For certain patients, anti-inflammatory medication and/or subacromial injections of local anaesthetic and/or steroid may be helpful – although multiple injections (≥ 3 or 4) should, in general, be avoided 45 46
There are countless studies demonstrating successful ‘conservative’ (i.e. Physical Therapy) management for RTC disease and RTC tears. The vast majority of cases of RTC Tendonitis, Tendinosis and/or Impingement Syndrome will experience good results with a proper Physical Therapy approach.
A Comprehensive Physical Therapy Approach will
- Improve the coordination of shoulder girdle muscles – This may include re-learning basic movement patterns to eliminate learned compensations. The objective is to restore the natural ‘Scapulo-Humeral Rhythm’ that defines proper shoulder movement. 47
- Improve strength and endurance of the RTC muscles individually, and their ability to work as a unit with more complex movements. 48
- Improve the strength and endurance of muscles that surround the scapula and shoulder girdle. Weakness of these surrounding muscles can increase stress to the RTC.
- Reducing Pain and Muscle Tension of Scapular and Neck Muscles – Exaggerated ‘tone’ or tension in certain muscles around the shoulder girdle can increase pain and impair the proper movement of the shoulder girdle. 49
Your Physical Therapist will likely incorporate some soft tissue massage and, perhaps some Trigger Point Dry Needling (TDN) techniques to reduce excessive tension in these, and other areas.
- Restoring a ‘balance’ of muscle strength and mobility of select muscles across the shoulder and scapula (and perhaps even cervical and thoracic spine) in order to restore proper postural alignment.
- Improving the mobility of the joints of the shoulder girdle, neck, upper back and rib cage. Mobilization or Manipulation of these joints can be effective at improving shoulder pain. 50 51
A consensus exists that Physical Therapy is the starting point for nearly all forms of RTC disease, and may patients will not require further treatment / intervention.
As stated previously, a multitude of evidence exists for the success of Physical Therapy in the short term for Shoulder pain, including RTC tears. However, the is some question regarding:
1) When it is appropriate to move more quickly towards a surgical approach; and
2) If there are potentially negative long-term consequences to avoiding a surgical repair if a tear is evident on films, but a patient is responsive to PT (i.e. is no longer painful and has regained function)
Some research has been done to look at longer-term outcomes for patients with RTC tears treated conservatively.
- Itoi et al reported on 54 patients with a total of 62 cuff tears, with mild pain and minimal functional deficit (e.g. they had not yet lost significant use of their arm). They found that 72% of these patients had ‘good’ or ‘excellent’ results, with an average follow up of 3.4 years 52
- Bokor et al reported that 74% of patients with confirmed rotator cuff tears, managed conservatively, had minimal or no pain at seven years and 86% were ‘satisfied’ with their result. 53
Not surprisingly, the ‘susscess rate’ of conservatively treated patients decreases with increased severity of damage. Bartolozzi et al found that patients were less likely to have a successful outcome with conservative management if they had pain and:
– A ‘full-thickness’ tears > 1cm2
– Symptoms persisting more than 1 year
– Significant functional impairment and weakness (determined by clinical testing) 54
The same author found that the following factors did not influence outcomes with conservative treatment
– Age
– Occupation
– Gender
– Instability (determined by testing)
– Arm Dominance
– Available Range of Motion (how far the patient could move their arm at initial assessment)
We have evidence that many people live with tears in their RTC – often with no pain or indication that there is an issue whatsoever.
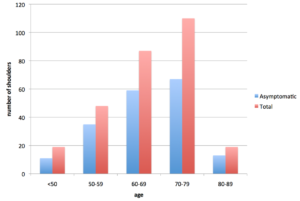
One compelling study investigated subjects in a Japanese mountain village. They found 283 full-thickness RTC tears (in 211 individuals) on Ultrasound imaging. 65.4% of these had no pain and reported no difficulty performing their daily activities. 55 They found that, in the general population, two-thirds of all RTC tears cause no symptoms.
We also know that individuals can function at a high level with RTC tears.
One study of twenty elite overhead athletes (12 college baseball pitchers and 8 professional tennis players) looked at the prevalence of MRI findings in asymptomatic (and high performing) athletes (average age of 26 years old). 56 The authors report that ‘none’ of the athletes had ‘normal’ MRI readings on their dominant shoulder – every single report found some degree of damage. 40% (8 out of 20) reported findings of partial or full-thickness RTC tears. That’s worth repeating: 40% of high performing, pain free shoulders in elite level athletes under the age of 30 had a RTC tear! The authors also found joint swelling (edema) in 90% of the MRIs, and subacromial swelling in 47.5%. To make sure they were not simply ‘finding’ tears that were about to turn painful, they followed these patients for 5 years. They report that “none of the interviewed athletes had subjective symptoms or had required any evaluation or treatment for shoulder related problems during the 5-year study period”. All athletes continued to participate in sports pain free. Compelling!
It should be noted that overhead athletes are unique. Other studies investigating the prevalence of MRI findings in asymptomatic professional baseball pitchers have demonstrated a wide spectrum of common findings unique to this group 57 While multiple studies have confirmed the existence of RTC disease and partial thickness tears of asymptomatic shoulders 58 59 other studies have questioned the existence of full-thickness tears as an incidental finding in the ‘young’ (less than 50 years) and active population. 60
However, there is evidence that RTC repair surgeries have better outcomes when repair is performed ‘early’. 61 62 Also, there is also some indication that if you have a painful RTC tear, and conservative management is unsuccessful at decreasing pain and returning function, there may be negative consequences to avoiding surgery.
These consequences include:
– An increase in tear size
– “Retraction” of the tear (i.e. further pulling away from the proper
attachment point); and/or
– A degradation of tissue quality leading to difficulty of future repair 63 64 65 66 67
The ‘worst case’ scenario is for the tear to progress to an “irreparable rotator” tear. Tears may be determined to be irreparable if they are of particularly large size, with significant tendon retraction and poor tissue quality (muscle atrophy with fatty infiltration). In these cases, patients may go on to develop secondary degenerative changes of the glenohumeral joint termed “rotator cuff arthropathy (Joint Degeneration).” 68
In the case of continued pain and poor function after 2-3 months of conservative treatment, including proper Physical Therapy, your orthopedic surgeon may order an Ultrasound Image or MR Imaging to visualize the damage. An ultrasound scan can offer a “dynamic” assessment of the rotator cuff with less expense than an MRI – however, the diagnostic value of these scans vary significantly based on the experience of the clinician performing them. 69 An MRI largely remains the ‘gold standard’ to visualize the tear location, size, extent of retraction and the amount of ‘fatty infiltration’ or atrophy of the RTC muscles. ‘Fatty infiltration’ is a degenerative process by which muscle tissue is replaced with adipose tissue. It is commonly found in long standing RTC disease and it’s presence has a strong influence on the success rate and ultimate outcome following surgical repairs. 70 71 72 73
Taking all of these factors into consideration, the decision to have, or not to have, a surgical repair of the RTC is complex. Findings that may suggest the indication for surgical intervention include:
- 3-4 Months of ‘failed’ conservative treatment including Physical Therapy and at least 1 subacromial injection
- A RTC Tear > 1 inch
- Significant pain and/or weakness
- Progressive (deteriorating) functional loss 74
- Work and/or Sports participation places high demands on the shoulder
Surgery
Surgeries for RTC tears have increased in frequency across the country even though the guidelines for performing these repairs remains uncertain. ‘Indications’ for surgical intervention must be tailored to the unique situation and needs of the individual.
Consider the following:
- Conservative Vs Surgery for RTC Tears?
In cases of smaller tears surgery may not be necessary / may not provide additional value beyond conservative (Physical Therapy) treatment.
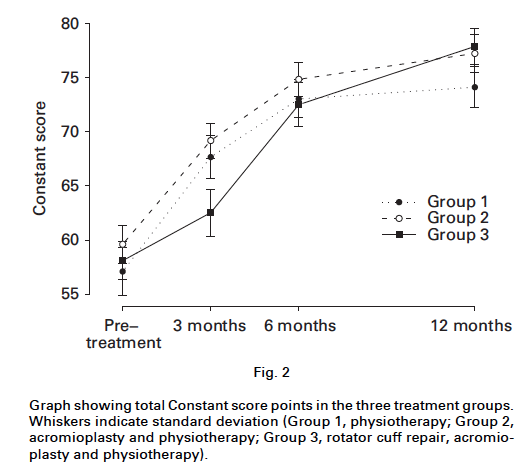
The graph below is from a study of 180 patients (age > 55 years) with non-traumatic Supraspinatus tears. They compared the outcomes for patients broken up into 3 groups: 1) Physical Therapy alone, 2) Physical Therapy + Acromioplasty and, 3) Surgical RTC repair + Acromioplasty + Physical Therapy. 75
The authors found no difference in long-term (1 year) outcome between groups. The only significant difference in short-term outcomes was a worse score for the ‘Repair Group’ at 3 months.
Interestingly Patient satisfaction was better in the surgical group at 1 year – 87% in the PT group, 96% in the acromioplasty and PT group, and 95% in the cuff repair and acromioplasty and PT group. The authors conclude that “with the exception of an acute traumatic cuff tear that results in an abrupt loss of strength, there is usually an opportunity for non-operative management. This is particularly the case for partial thickness tears.”
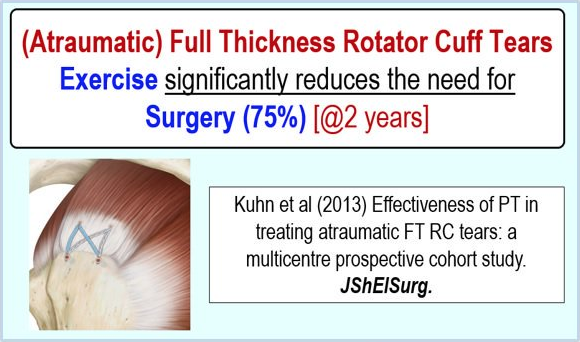
Conservative care (Physical Therapy) can also be very effective for non-traumatic full-thickness RTC tears (in patients > 60 year old) eliminating the ‘need’ for surgery in approximately 75% of individuals. 76
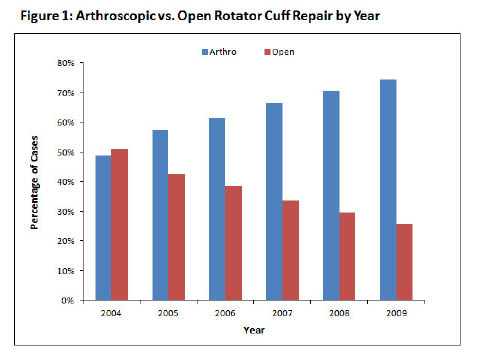
From 1996 > 2006 the rates of surgical repairs for RTC tear rose dramatically. A 34% increase in open repairs, and a 600% increase in arthroscopic repairs. While this is in part due to improved techniques and sophistication of arthroscopic technology, the trend also likely reflects increasing access to advanced imaging techniques such as contrast MRI or shoulder ultrasound, that can reveal small defects in the rotator cuff tendons, which may, or may not be largely related to the patients pain.
Armed with the knowledge that
1) RTC Tears are common and frequently asymptomatic
AND
2) Tears are being ‘found’ with greater regularity
We must be careful not to intervene on every ‘finding’. In other words – Don’t ‘fix’ it just because it’s there.
I’ve heard this referred to as the Mallory Justification – George Mallory was a British mountaineer who attempted the first ascent of Mount Everest. When asked why he wanted to climb Everest, he replied, “because it’s there”… Mallory did not return from his ill-fated attempt.
Decisions to, or not to, have surgery are complex and multifactorial. Experts in the field aknowledge that “there are no reliable methods of predicting successful outcomes.” 77
For this reason, there is no ‘fool proof’ algorithm one can follow to determine if they are perusing the right course. There are instances where the ‘correct’ course is clear – a young, healthy athlete suffers a traumatic, and acute tear of one of the RTC muscles. Almost certainly, surgery will be indicated – and quickly. On the other end of the spectrum, a gradual, ‘degenerative’ tear in a muscle with very little structural integrity may be deemed ‘un-repairable’. Surgical ‘repair’ is not even an option. Most cases, however, fall somewhere in the middle of the spectrum.
Here, the advice and counsel of a shoulder expert – an Orthopedic Surgeon – is critical. Your current clinical presentation, injury history, general health and activity level all factor into the decision – regardless of how much ‘damage’ the images reveal (with few exceptions).
Often, surgeons will request (appropriately!) a course of Physical Therapy. If the shoulder is responsive to conservative care, surgery may not be ‘needed’. Many patient will say – “yes, it’s getting better, but it will never heal on it’s own without surgery, right?” Consider, there is much more to being happy with the use of your shoulder than it’s ‘appearance’ on an MRI. (Also, see below regarding ‘failure’ rates)
When Strength, mobility and function improve with Physical Therapy, surgery may not be the best route.
Also, get a second opinion if you have any doubts. You don’t want to go into a surgery like a RTC repair with doubts and reservations. The recovery is long and the rehabilitation is extensive.
Last, educate your self on process… Not all surgical interventions are the same.
Open Vs Arthroscopic Repairs?
Traditionally, RTC repairs were made under an ‘open’ incision. The first ever, was performed by Dr. Codman in 1911
This caused significant muscle damage and recovery was slower and more painful. With the advent of arthroscopic techniques in the 80’s the outcomes improved and complication rates lowered.
Currently, RTC repairs are performed ‘Open’, ‘Mini-Open’ or ‘Arthroscopically’. The reasons for choosing which are multifactorial. Overall, as technology and arthroscopic techniques improve, an overall shift has occurred away from open repairs.
Open repair
A traditional open surgical incision is utilized less frequently today, but may be necessary under specific conditions, usually with massive or complex tears with significant (>2cm) retraction of the muscle away from it’s original attachment site.
This approach includes ‘splitting’ the deltoid muscle (the large, easily visible muscle covering the shoulder joint) from the tip of the shoulder laterally ~ 3-5cm to access the area for repair. 78 79 80
Outcomes for open repairs are mostly favorable. Studies show ~ 80% – 94% of patients experience ‘good’ to ‘excellent’ results. 81 82 83 84
We must keep in mind, however, most patients who undergo open repairs are in pretty bad shape before the intervention. ‘Good’ outcomes do not necessarily mean a return to high-level use, it means a return to basic function and a (hopefully) large reduction in pain severity. For example, Hawkins et al, followed a 100 of his patients for more than 4 years after surgery. The ‘Abduction’ range of motion (the ability to raise the arm away from the side) improved (on average) from 81 degrees (unable to raise arm to shoulder level) to 125 degrees. This is both positive and meaningful. However, full overhead range requires ~ 160 degrees. 85
Other research has shown that outcomes are most largely effected by ‘tear size’, with ‘large’ and ‘massive’ tears having more modest success rates. Currently, open repairs are reserved almost exclusively for these larger tears (as smaller tears tend to be repairable arthroscopically). 86
The point being, the surgery has a fairly high success rate when patient selection is judicious, however, expectations should be somewhat tempered.
Disadvantages of open repairs are mainly a result of the splitting of the deltoid muscle. This results in more post-operative pain, a slower progression of rehabilitation and a risk of long standing deltoid dysfunction, which is rare, but potentially devastating. 87 88 89 90 91 92
Mini-Open Repair
New techniques and instruments allow surgeons to perform more extensive repairs through a small incision (generally ~ 2 to 6 cm) in the deltoid muscle. This allows access to the shoulder without full deltoid ‘take down’ (e.g. the deltoid origin and insertion are undisturbed).
Mini-Open repairs are indicated for Medium to Large tears without significant retraction (less than 2 cm) of the muscle away from it’s original attachment site. 93 94 95 96
The mini-open approach has been shown by multiple studies, to provide consistent outcomes similar to open-repairs, but with less post-operative pain and a reduced complete immobilization period. Overall, long-term success rates are high with 80-88% of patients reporting ‘good’ to ‘excellent’ results. 97 98 99
Arthroscopic Repair
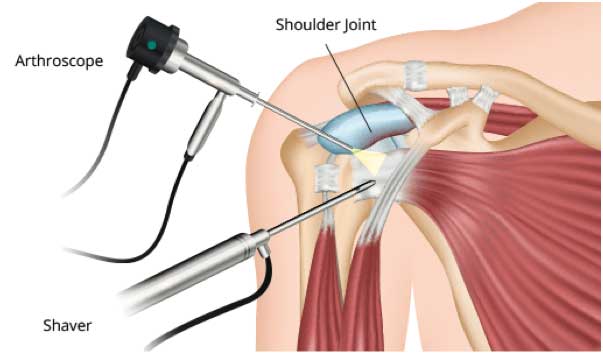
An optical scope and small instruments are inserted through small puncture wounds instead of through a larger incision. The operation is carried out under visual control via a video display.
Arthroscopic repairs are utilized for small to medium size tears (and some larger tears) with minimal ‘retraction’ of the muscle away from it’s original attachment site and ‘good’ tissue quality. 100 101 102
Proponents of arthroscopic techniques point to the lower risk of complications such as stiffness, infection, and deltoid disruption. However, critics point out limited long-term outcomes data and the technical difficulty associated with this procedure (there is a steep learning curve for arthroscopic RTC repairs).
Also, there is some controversy over the ‘optimal’ fixation technique. (* See box below)
Outcomes
Although ‘full open’ repairs (Large incision) are rare these days, there remains some debate and disagreement as to the superiority of arthroscopic vs. mini-open repair techniques. A recent systematic review by Lindley and Jones found no statistically significant difference in re-rupture rates or outcomes when comparing arthroscopy vs. a mini-open repair technique. The only difference found (aside from a smaller scar) was decreased post-operative pain, in the short-term, for patients who underwent arthroscopic repair. 103
Another review (meta-analysis) 104 from 2008 had similar findings – no difference in outcomes or complication rates.
Conflicting evidence was published in 2009 by Colegate-Stone et al. 105 They performed a prospective comparative study, looking at 123 consecutive patients who underwent rotator cuff repairs (both arthroscopic and mini-open repairs) and found that arthroscopic repairs consistently displayed slightly better outcomes.
In general, the arthroscopic and mini-open repairs allow for a quicker, earlier start to rehabilitation and cause less post-operative pain, as compared to more traditional approaches. 106
Arthroscopy may provide additional benefits beyond the mini-open procedures, but this remains to be proven.
Overall, repair of a RTC tear provides fairly predictable functional improvement and good overall patient satisfaction. 107 Results are ‘generally favorable’. 108 and report a high percentage of Excellent or Good results following surgery (~ 60 to 85%).
Complications
Of concern, is the ‘complication’ and ‘re-rupture’ rate.
Approximately 38% of all patients undergoing a surgical repair experience some sort of ‘complication’ 109 Even more concerning is the re-rupture rates. The literature is highly variable here. General population rates range from 13% 110 to 68% 111 When looking solely at ‘large’ tears, the re-rupture rate increases to between 41% and 94% 112 113 114
Failure Rates of Surgical Repair:
• 35% of all tears fail
• 20% of supraspinatus repairs fail
• 50% of repairs of two tendons fail
• 68% of repairs of three tendons fail
• 25% of repairs to people aged 34-55 fail
• 35% of repairs to people aged 56-70 fail
• 45% of repairs to people aged 71-85 fail 115 116
Surgical repair of a previously failed surgery has less favorable outcomes, with a ~ 69% ‘satisfaction’ rate (despite often modest expectations) 117
*** Interestingly, most patients who experience a re-rupture will none-the-less report improvements in pain and function. Perhaps due to the rehabilitation?! *** 118
Techniques
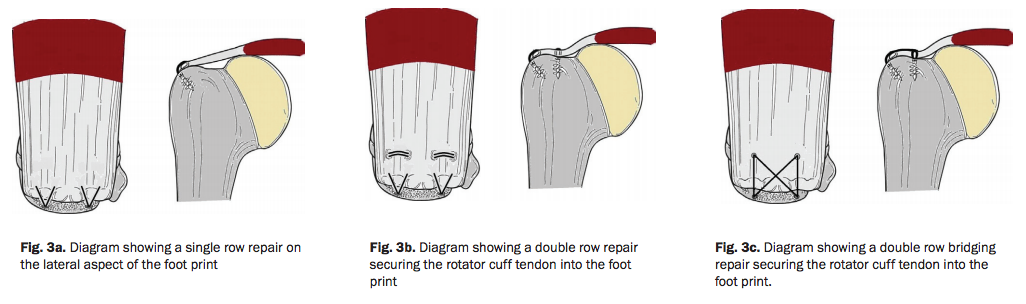
In addition to the ‘approach’ used, the specific ‘technique’ may effect the outcome of the repair. These different repair techniques include single row, double row, or ‘suture bridge’ repairs.
Traditionally, repairs were made with a ‘single row’ of anchors. More recently, criticism of this approach suggested that the smaller contact created with a single row does not accurately re-create the natural ‘footprint’ of the original muscle attachment. Double row and suture bridge techniques, as well as techniques utilizing a combination of sutures and transosseous screws, have attempted to address this issue. These techniques have been shown to restore 100% of normal contact area of the supraspinatus footprint (attachment) – compared to 65% for single-row fixation. 119 120
Also, biomechanical studies demonstrate an improved ‘load to failure’ strength allowing for an earlier initiation of rehabilitation. 121 122 123 124
Some earlier studies have shown similar results in terms of outcomes and failure rates with all techniques. 125 126
However, a more recent review demonstrated a significant difference in the re-rupture rate when comparing a single row repair (20% re-rupture) to double row suture bridge techniques (7% re-rupture). 127
Additionally, some studies have found that patients undergoing repairs with double row or transosseous techniques experience less pain, demonstrate better early motion, and report higher overall initial satisfaction. 128
Post-Op Rehab
The speed of progression through the various rehabilitation “stages” depends on many factors, including:
- Size of the repair
- Surgical technique (open vs. arthroscopic; single-row vs. double row, etc)
- Location of the tear (precautions will differ between ‘Type A” and “Type D” tears)
- Shape of the tear (tears with ‘retraction’ will be rehabbed more cautiously)
- ‘Acuteness’ (‘fresh’ injuries tend to have better tissue quality, but be at risk of post-operative stiffness. Thus, they may be rehabbed more aggressively)
- Tissue quality (atrophy, fatty infiltration)
- Patient specific factors (age, overall health, etc)
- Patient specific goals (sedentary lifestyle vs. athlete or manual laborer)
As you can see from the above list – Rehab from shoulder surgery is highly variable.
Patients experience widely differing levels of post-operative pain, stiffness and expectations are variable depending on many, many factors.
Below are some rough estimates in recovery times to guide expectations. Please note – there is significant variation that is expected and alterations from these estimates do not indicate a ‘poor’ recovery or portend a sub-optimal outcome.
- Immediate Post-Operative Phase (Day 1-10)
- Protective Phase (Week 2 – Week 6)
- Intermediate Phase (Week 7 – Week 14)
- Advanced Strengthening Phase (Week 15 – Week 22)
- Return to Activity Phase (Week 23 – Week 36)
Immediate Post-Op & Protection phase
The initial phase after surgery focuses on:
– Protecting the repair
– Promoting initial healing
– Preventing excessive shoulder stiffness
Protection of the repair is essential as the tendon to bone interface ‘heals’ slowly. Vascularized fibrous adhesions are initially created to begin this process. 129
At least 12 weeks of healing time is required to return adequate ‘pull-out’ strength to the repair. Initial immobilization is essential to allow early healing to take place undisturbed. This typically requires the use of an ‘abduction’ sling with the shoulder positioned slightly in front of the mid-line of the body (in the ‘scapular’ plane) and the elbow ~ 30-45° away from the side. This places the least amount of strain on the healing repair (assuming a supraspinatus repair) 130 and allows for the greatest amount of blood flow to the repaired tendon. 131
Ice will be used regularly during the initial phases as it has been shown to decrease pain levels, improve sleep and allow for more full participation in rehabilitation during this early phase.
During this phase “Passive” Range Of Motion (ROM) exercises (where the shoulder muscles are relaxed) may be used to prevent excessive stiffness. This is performed within specific ranges that place little stress on the repair. 132 Note – during this initial post-op phase, this should not be painful stretching; only movement of the shoulder through the available arc.
During this phase, the patient will also begin exercises to mobilize the surrounding joints – the neck, shoulder blade, elbow, forearm and wrist joints & muscles.
Firing the muscles around the shoulder may, towards the later portion of this phase, be performed lightly and isometrically – meaning the arm is fixed against the wall or door frame while the patient lightly pushes into an immovable object. The muscles turn on gently, but the joint does not move. (This is introduced to small and/or partial repairs with healthy tissue. Otherwise, this is delayed until week 6-8)
Exercises (such as gentle isometrics) are not done in this stage to ‘build strength’. Rather, intermittent activation of the muscle assists in facilitating a healing environment – ‘mobilizing’ or stimulating activity in the cells surrounding the surgical site that assist in initial healing process. Gentle, ‘cyclic’ loading can also promote recapillarization (returning blood flow) to the healing area 133 and facilitate proper fiber alignment at the injury site 134 – Loading for ‘strength gains’ comes later.
During this early / protective phase, pool rehab may be started (typically ~ 3-4 weeks out). The water reduces the weight of the limb, decreasing the amount of RTC work needed to elevate the arm, and thus allowing earlier movement with reduced strain to healing tissues. 135 This is particularly helpful in patients who are recovering from a large or massive tear and must ‘protect’ the repair for longer periods. It is also helpful with patients who experience a significant amount of post-operative pain and / or stiffness. An aquatic program has been shown to help accelerate the return of shoulder elevation ROM. 136
Depending on the size of the tear (as well as the list of influencing factors listed above) the ‘protective phase’ may last 3-4 weeks on the short end, or up to 10 weeks on the high end (e.g. large or massive tears with suspect tissue quality). 137
Intermediate Phase
On average, this ‘phase’ begins ~ Week 6 or 7 (but will vary as previously stated) and lasts until ~ 3-4 months after surgery.
The sling is no longer in use, and patients begin returning to normal ‘daily use’ of the arm for light tasks (dressing, driving, eating, etc.)
Especially in the earlier weeks of this stage, the repair still needs to be protected to some degree. It is about 40% as strong as a ‘normal’ tendon at eight weeks and about 60% as strong at 12 weeks. 138
There is some inconsistency in the literature here. Some studies have shown full healing with return of ‘final tensile strength’ by 12-16 weeks. Other studies have shown a slower timetable.
Animal model studies suggest a much longer ‘healing’ timetable of ~ 26 weeks. 139 140
This is likely highly variable depending on multiple factors including tissue health and early rehabilitation approach.
For this reason, exercises are introduces at low loads to further encourage the healing process. Light resistance / strengthening exercises may include:
– Manual Resistance Exercises (the Physical Therapists resisting you with their hands)
– Light tubing or Pulley exercises
– Gentle partial weight bearing activities
* Heavy lifting, Resisted overhead motions and Challenging weightbearing activities are deferred during this period.
The goal of exercises performed in this phase is to gradually return loads to the repaired tissue. This should be done in a slow progressive/graded fashion to encourage ‘remodeling’ of the repair.
Electrical stimulation may be utilized to improve the ‘recruitment’ of some of the RTC muscles during this phase. Often these muscles remain ‘inhibited’ from the pain and/or swelling associated with the surgery – however, it is now safe for them to ‘wake up’.
Neuromuscular electrical stimulation (NMES) has been shown to significantly improve RTC strength return when combined with an appropriate rehab protocol. 141
Also, during this phase the shoulder blade muscles should be challenged. It is safe to perform exercises that feel very difficult to the muscles around the shoulder blade, without stressing the RTC, if done properly.
Most patients will need to continue to work on their ROM, increasing the ‘aggressiveness’ during this phase. By the end of the intermediate phase, full ROM is the goal.
If shoulder stiffness is slow to recover – your Physical Therapist has a host of tools available to assist you. First and foremost, the patient must be compliant with regular / consistent ROM program at home. The first step with ‘stubborn’ stiffness is not to go harder, but rather more frequent with these mobilization exercises. In addition to this, your PT may: massage the muscles adjacent to the shoulder, perform joint mobilization maneuvers, and/or utilize instruments to mobilize fascial restrictions or fibrosis adjacent to the repair 142
Advanced Strengthening Phase
The Advanced Strengthening Phase begins ~ 3 months after surgery and typically lasts until ~ 6 months out.
At this point the repair is ‘fairly solid’ (though not quite 100%) and the patient has regained their ROM (hopefully). They are typically able to return to basic ‘activities of daily living’ with some restrictions (typically heavy and/or overhead lifting).
What remains to be done is to 1) Regain full strength & endurance, and 2) Fine-tune the neuro-muscular control and coordination of the shoulder and adjacent structures (Neck, Shoulder Blade, Upper Back, etc) with challenging tasks.
During this phase the patient progresses to harder, heavier and more ‘dynamic’ tasks – including overhead, ‘dynamic’ (moving) weightbearing, and (if appropriate) plyometric exercises.
Expect to continue with ROM exercises to ‘fine-tune’ end-range mobility and maintain current gains. If ROM is not 100%, during this phase, the Physical Therapist will begin to mobilize the shoulder joint more aggressively.
During this phase, the patient should be performing 30-45 minutes of upper body exercises at a minimum, during their Physical Therapy sessions. Typically, this would be complemented with a home strengthen exercise program to ensure appropriate frequency and weekly volume.
Return to Activity Phase
Return to Activity Phase typically begins around month 5-6 after surgery.
This ‘phase’ is highly individualized. For example, a 60 year old with a sedentary job who walks for exercise, will not need much of a ‘return to activity phase’. Once they have full ROM and 80-90% of their strength back, they can pretty much do anything they want to do. However, a 38 year-old baseball player, or a 45 year-old manual laborer will require more extensive rehab.
This phase focuses on high-level strength, overhead endurance, introducing work and/or sports-specific demands as well as (if appropriate) a graded return to throwing interval program.
Returning to 100% strength and overhead endurance is a significant undertaking. For patients with a high activity level, if they do not return ROM to 100%, fine-tune their coordination with challenging tasks, and get strength and endurance up to a high-level, they risk re-injury the shoulder, or perhaps neighboring segment (e.g. the neck).
This phase can take an additional 3-6 months (or even more).
If return to sport is desired, your Physical Therapist will perform a detailed assessment of strength and ability to perform sports-specific tasks to ensure readiness. This assessment is critical to give your surgeon necessary information on which to base your return to sports clearance.
With diligence and a proper rehab program, maximal improvement will still not be evident until 6 > 16 Months out from surgery (depending on variables discussed previously) 143
This is not to say that patients don’t feel ‘better’ much sooner than that, but ‘full function’ takes quite a bit of time, and work!
- Bot, S. D., Van der Waal, J. M., Terwee, C. B., Van der Windt, D. A. W. M., Schellevis, F. G., Bouter, L. M., & Dekker, J. (2005). Incidence and prevalence of complaints of the neck and upper extremity in general practice. Annals of the rheumatic diseases, 64(1), 118-123.
- Luime, J. J., Koes, B. W., Hendriksen, I. J. M., Burdorf, A., Verhagen, A. P., Miedema, H. S., & Verhaar, J. A. N. (2004). Prevalence and incidence of shoulder pain in the general population; a systematic review. Scandinavian journal of rheumatology, 33(2), 73-81.
- Lewis JS. Rotator cuff tendinopathy/subacromial impingement syndrome: is it time for a new method of assessment? Br J Sports Med 2009;43(4):259-64
- H.Horng-Chaung, Influence of rotator cuff tearing on glenohumeral stability, Journal of Shoulder and Elbow Surgery, 1997, pp 413 – 422, (2B), (E)
- Lohr JF, Uhthoff HK. The microvascular pattern of the supraspinatus tendon. Clin Orthop 1990;254:35-8.
- Rathbun JB, Macnab I. The microvascular pattern of the rotator cuff. J Bone Joint Surg [Br] 1970;52-B:540-53.
- Rothman RH, Parke WW. The vascular anatomy of the rotator cuff. Clin Orthop 1965;41:176-86.
- Thompson WO, Debski RE, Boardman ND III, et al: A biomechanical analysis of rotator cuff deficiency in a cadaveric model. Am J Sports Med 1996;24:286-292.
- McGough RL, Debski RE, Taskiran E, Fu FH, Woo SL: Mechanical properties of the long head of the biceps tendon. Knee Surg Sports Traumatol Arthrosc 1996;3:226-229.
- Mitchell, C., Adebajo, A., Hay, E., & Carr, A. (2005). Shoulder pain: diagnosis and management in primary care. Bmj, 331(7525), 1124-1128.
- Harkness, E. F., MacFarlane, G. J., Nahit, E. S., Silman, A. J., & McBeth, J. (2003). Mechanical and psychosocial factors predict new onset shoulder pain: a prospective cohort study of newly employed workers. Occupational and environmental medicine, 60(11), 850-857.
- Van Der Windt, D. A., Thomas, E., Pope, D. P., De Winter, A. F., Macfarlane, G. J., Bouter, L. M., & Silman, A. J. (2000). Occupational risk factors for shoulder pain: a systematic review. Occupational and environmental medicine, 57(7), 433-442.
- Vandvik, P. O., Lähdeoja, T., Ardern, C., Buchbinder, R., Moro, J., Brox, J. I., … & Noorduyn, J. (2019). Subacromial decompression surgery for adults with shoulder pain: a clinical practice guideline. BMJ, 364, l294.
- Ide K, Shirai Y, Ito H, Ito H: Sensory nerve supply in the human subacromial bursa. J Shoulder Elbow Surg 1996;5:371-382.
- Soifer TB, Levy HJ, Soifer FM, Klein- bart F, Vigorita V, Bryk E: Neurohistology of the subacromial space. Arthroscopy 1996;12:182-186.
- Haahr JP OS, Dalsgaard J, Norup K, Frost P, Lausen S, et al. Exercises versus arthroscopic decompression in patients with subacromial impingement: a randomised, controlled study in 90 cases with a one year follow up. Annals of the Rheumatic Diseases. 2005;64(5):760-764.
- Brox JI SP, Ljunggren AE, Brevik JI. Arthroscopic surgery compared with supervised exercises in patients with rotator cuff disease (stage 11 impingement syndrome). British Medical Journal. 1993;307:899-903.
- Rahme H S-BE, Westerberg CE, Lundberg E, Sorensen S, Hilding S. The Subacromial Impingement Syndrome. A study of results of treatments with special emphasis on predictive factors and pain generating mechanisms. Scandinavian Journal of Rehabilitation Medicine. 1998;30:253-262.
- Ketola, S., Lehtinen, J., Rousi, T., Nissinen, M., Huhtala, H., Konttinen, Y. T., & Arnala, I. (2013). No evidence of long-term benefits of arthroscopic acromioplasty in the treatment of shoulder impingement syndrome. Bone and Joint Research, 2(7), 132-139.
- Ketola, S., Lehtinen, J., Arnala, I., Nissinen, M., Westenius, H., Sintonen, H., … & Rousi, T. (2009). Does arthroscopic acromioplasty provide any additional value in the treatment of shoulder impingement syndrome?. Bone & Joint Journal, 91(10), 1326-1334.
- Ketola, S., Lehtinen, J., Arnala, I., Nissinen, M., Westenius, H., Sintonen, H., … & Rousi, T. (2010). Arthroscopic decompression with acromioplasty and structured exercise was no more effective and was more expensive than exercise alone. The Journal of Bone and Joint Surgery, 92(10), 1999.
- Vandvik, P. O., Lähdeoja, T., Ardern, C., Buchbinder, R., Moro, J., Brox, J. I., … & Noorduyn, J. (2019). Subacromial decompression surgery for adults with shoulder pain: a clinical practice guideline. BMJ, 364, l294.
- Vandvik, P. O., Lähdeoja, T., Ardern, C., Buchbinder, R., Moro, J., Brox, J. I., … & Noorduyn, J. (2019). Subacromial decompression surgery for adults with shoulder pain: a clinical practice guideline. BMJ, 364, l294.
- Aydin, A., Yildiz, V., Kalali, F., Yildirim, O. S., Topal, M., & DOSTBil, A. (2011). The role of acromion morphology in chronic subacromial impingement syndrome. Acta Orthopaedica Belgica, 77(6), 733.
- Henkus, H. E., De Witte, P. B., Nelissen, R. G. H. H., Brand, R., & Van Arkel, E. R. A. (2009). Bursectomy compared with acromioplasty in the management of subacromial impingement syndrome. Bone & Joint Journal, 91(4), 504-510.
- Donigan, J. A., & Wolf, B. R. (2011). Arthroscopic subacromial decompression: acromioplasty versus bursectomy alone-does it really matter? A systematic review. The Iowa orthopaedic journal, 31, 121.
- Papadonikolakis, A., McKenna, M., Warme, W., Martin, B. I., & Matsen, F. A. (2011). Published evidence relevant to the diagnosis of impingement syndrome of the shoulder. J Bone Joint Surg Am, 93(19), 1827-1832.
- Vandvik, P. O., Lähdeoja, T., Ardern, C., Buchbinder, R., Moro, J., Brox, J. I., … & Noorduyn, J. (2019). Subacromial decompression surgery for adults with shoulder pain: a clinical practice guideline. BMJ, 364, l294.
- Whittle, S., & Buchbinder, R. (2015). Rotator cuff disease. Annals of internal medicine, 162(1), ITC1-ITC1.
- Arroll, B., & Goodyear-Smith, F. (2005). Corticosteroid injections for painful shoulder: a meta-analysis. Br J Gen Pract, 55(512), 224-228.
- Littlewood, C., May, S., & Walters, S. (2013). A review of systematic reviews of the effectiveness of conservative interventions for rotator cuff tendinopathy. Shoulder & Elbow, 5(3), 151-167.
- Littlewood, C., May, S., & Walters, S. (2013). A review of systematic reviews of the effectiveness of conservative interventions for rotator cuff tendinopathy. Shoulder & Elbow, 5(3), 151-167.
- Lädermann, A., Denard, P. J., & Collin, P. (2015). Massive rotator cuff tears: definition and treatment. International orthopaedics, 39(12), 2403-2414.
- Lädermann, A., Denard, P. J., & Collin, P. (2015). Massive rotator cuff tears: definition and treatment. International orthopaedics, 39(12), 2403-2414.
- Sher, J.S.,Uribe, J.W., Posada, A., Murphy, B.J., & Zlatkin, M.B. (1995). Abnormal findings on magnetic resonance images of asymptomatic shoulders. J Bone Joint Surg Am, 77(1), 10-15.
- Yamaguchi, K., Tetro, A. M., Blam, O., Evanoff, B. A., Teefey, S. A., & Middleton, W. D. (2001). Natural history of asymptomatic rotator cuff tears: a longitudinal analysis of asymptomatic tears detected sonographically. Journal of Shoulder and Elbow Surgery, 10(3), 199-203.
- Yamanaka, K., & Matsurnoto, T. (1994). The Joint Side Tear of the Rotator Cuff: A Followup Study by Arthrography. Clinical orthopaedics and related research, 304, 68-73.
- Yamaguchi, K., Tetro, A. M., Blam, O., Evanoff, B. A., Teefey, S. A., & Middleton, W. D. (2001). Natural history of asymptomatic rotator cuff tears: a longitudinal analysis of asymptomatic tears detected sonographically. Journal of Shoulder and Elbow Surgery, 10(3), 199-203.
- Fukuda, H., Hamada, K., Nakajima, T., & Tomonaga, A. (1994). Pathology and pathogenesis of the intratendinous tearing of the rotator cuff viewed from en bloc histologic sections. Clinical orthopaedics and related research, 304, 60-67.
- Fucentese, S. F., Andreas, L., Pfirrmann, C. W., Gerber, C., & Jost, B. (2012). Evolution of nonoperatively treated symptomatic isolated full-thickness supraspinatus tears. The Journal of Bone & Joint Surgery, 94(9), 801-808.
- Clement, N. D., Jenkins, P. J., Brenkel, I. J., & Walmsley, P. (2012). Predictors of mortality after total knee replacement. J Bone Joint Surg Br, 94(2), 200-204.
- Bartolozzi, A., Andreychik, D., & Ahmad, S. (1994). Determinants of outcome in the treatment of rotator cuff disease. Clinical orthopaedics and related research, 308, 90-97.
- Bokor, D. J., Hawkins, R. J., Huckell, G. H., Angelo, R. L., & Schickendantz, M. S. (1993). Results of nonoperative management of full-thickness tears of the rotator cuff. Clinical orthopaedics and related research, 294, 103-110.
- Itoi, E., & Tabata, S. (1992). Conservative treatment of rotator cuff tears. Clinical orthopaedics and related research, 275, 165-173.
- Clemnent N, McBirnie J. Focus on Rotator Cuff Tears. British Editorial Society of Bone and Joint Surgery. 2012.
- Fukuda, H. (2003). The management of partial-thickness tears of the rotator cuff. JOURNAL OF BONE AND JOINT SURGERY-BRITISH VOLUME-, 85(1), 3-11.
- Collin, P. G., Gain, S., Huu, F. N., & Lädermann, A. (2015). Is rehabilitation effective in massive rotator cuff tears?. Orthopaedics & Traumatology: Surgery & Research, 101(4), S203-S205.
- Ainsworth, R., & Lewis, J. S. (2007). Exercise therapy for the conservative management of full thickness tears of the rotator cuff: a systematic review. British journal of sports medicine, 41(4), 200-210.
- Collin, P. G., Gain, S., Huu, F. N., & Lädermann, A. (2015). Is rehabilitation effective in massive rotator cuff tears?. Orthopaedics & Traumatology: Surgery & Research, 101(4), S203-S205.
- Van der Hoeven, H., & Kibler, W. B. (2006). Shoulder injuries in tennis players. British journal of sports medicine, 40(5), 435-440.
- Oh, J. H., Yoon, J. P., Kim, J. Y., & Oh, C. H. (2010). Isokinetic muscle performance test can predict the status of rotator cuff muscle. Clinical Orthopaedics and Related Research®, 468(6), 1506-1513.
- Itoi, E., & Tabata, S. (1992). Conservative treatment of rotator cuff tears. Clinical orthopaedics and related research, 275, 165-173.
- Bokor, D. J., Hawkins, R. J., Huckell, G. H., Angelo, R. L., & Schickendantz, M. S. (1993). Results of nonoperative management of full-thickness tears of the rotator cuff. Clinical orthopaedics and related research, 294, 103-110.
- Bartolozzi, A., Andreychik, D., & Ahmad, S. (1994). Determinants of outcome in the treatment of rotator cuff disease. Clinical orthopaedics and related research, 308, 90-97.
- Yamamoto, A., Takagishi, K., Kobayashi, T., Shitara, H., & Osawa, T. (2011). Factors involved in the presence of symptoms associated with rotator cuff tears: a comparison of asymptomatic and symptomatic rotator cuff tears in the general population. Journal of shoulder and elbow surgery, 20(7), 1133-1137.
- Connor, P. M., Banks, D. M., Tyson, A. B., Coumas, J. S., & D’Alessandro, D. F. (2003). Magnetic resonance imaging of the asymptomatic shoulder of overhead athletes. The American journal of sports medicine, 31(5), 724-727.
- Miniaci, A., Mascia, A. T., Salonen, D. C., & Becker, E. J. (2002). Magnetic resonance imaging of the shoulder in asymptomatic professional baseball pitchers. The American journal of sports medicine, 30(1), 66-73.
- Chandnani, V., Ho, C., Gerharter, J., Neumann, C., Kursunoglu-Brahme, S., Sartoris, D. J., & Resnick, D. (1992). MR findings in asymptomatic shoulders: a blind analysis using symptomatic shoulders as controls. Clinical imaging, 16(1), 25-30.
- Sher, J. S., Uribe, J. W., Posada, A., Murphy, B. J., & Zlatkin, M. B. (1995). Abnormal findings on magnetic resonance images of asymptomatic shoulders. J Bone Joint Surg Am, 77(1), 10-15.
- Miniaci, A., Dowdy, P. A., Willits, K. R., & Vellet, A. D. (1995). Magnetic resonance imaging evaluation of the rotator cuff tendons in the asymptomatic shoulder. The American journal of sports medicine, 23(2), 142-145.
- Bassett, R. W., & Cofield, R. H. (1983). Acute Tears of the Rotator Cuff: The Timing of Surgical Repair. Clinical orthopaedics and related research, 175, 18-24.
- Cofield, R. H., Parvizi, J., Hoffmeyer, P. J., Lanzer, W. L., Ilstrup, D. M., & Rowland, C. M. (2001). Surgical repair of chronic rotator cuff tears. The Journal of Bone & Joint Surgery, 83(1), 71-71.
- Prasad, N., Odumala, A., Elias, F., & Jenkins, T. (2005). Outcome of open rotator cuff repair. An analysis of risk factors. Acta orthopaedica belgica, 71(6), 662.
- Ellman, H., Hanker, G., & Bayer, M. (1986). Repair of the rotator cuff. End-result study of factors influencing reconstruction. J Bone Joint Surg Am, 68(8), 1136-1144.
- Goutallier, D., Postel, J. M., Gleyze, P., Leguilloux, P., & Van Driessche, S. (2003). Influence of cuff muscle fatty degeneration on anatomic and functional outcomes after simple suture of full-thickness tears. Journal of Shoulder and Elbow Surgery, 12(6), 550-554.
- Goutallier, D., Postel, J. M., Bernageau, J., Lavau, L., & Voisin, M. C. (1995). Fatty infiltration of disrupted rotator cuff muscles. Revue du rhumatisme (English ed.), 62(6), 415-422.
- Thomazeau, H., Rolland, Y., Lucas, C., Duval, J. M., & Langlais, F. (1996). Atrophy of the supraspinatus belly assessment by MRI in 55 patients with rotator cuff pathology. Acta orthopaedica Scandinavica, 67(3), 264-268.
- Ecklund, K. J., Lee, T. Q., Tibone, J., & Gupta, R. (2007). Rotator cuff tear arthropathy. Journal of the American Academy of Orthopaedic Surgeons, 15(6), 340-349.
- Clemnent N, McBirnie J. Focus on Rotator Cuff Tears. British Editorial Society of Bone and Joint Surgery. 2012.
- Ellman, H., Hanker, G., & Bayer, M. (1986). Repair of the rotator cuff. End-result study of factors influencing reconstruction. J Bone Joint Surg Am, 68(8), 1136-1144.
- Goutallier, D., Postel, J. M., Gleyze, P., Leguilloux, P., & Van Driessche, S. (2003). Influence of cuff muscle fatty degeneration on anatomic and functional outcomes after simple suture of full-thickness tears. Journal of Shoulder and Elbow Surgery, 12(6), 550-554.
- Goutallier, D., Postel, J. M., Bernageau, J., Lavau, L., & Voisin, M. C. (1995). Fatty infiltration of disrupted rotator cuff muscles. Revue du rhumatisme (English ed.), 62(6), 415-422.
- Thomazeau, H., Rolland, Y., Lucas, C., Duval, J. M., & Langlais, F. (1996). Atrophy of the supraspinatus belly assessment by MRI in 55 patients with rotator cuff pathology. Acta orthopaedica Scandinavica, 67(3), 264-268.
- Sauerbrey, A. M., Getz, C. L., Piancastelli, M., Iannotti, J. P., Ramsey, M. L., & Williams, G. R. (2005). Arthroscopic versus mini-open rotator cuff repair: a comparison of clinical outcome. Arthroscopy: The Journal of Arthroscopic & Related Surgery, 21(12), 1415-1420.
- Kukkonen, J., Joukainen, A., Lehtinen, J., Mattila, K. T., Tuominen, E. K. J., Kauko, T., & Äärimaa, V. (2014). Treatment of non-traumatic rotator cuff tears. Bone Joint J, 96(1), 75-81.
- Kuhn, J. E., Dunn, W. R., Sanders, R., An, Q., Baumgarten, K. M., Bishop, J. Y., … & Ma, C. B. (2013). Effectiveness of physical therapy in treating atraumatic full-thickness rotator cuff tears: a multicenter prospective cohort study. Journal of Shoulder and Elbow Surgery, 22(10), 1371-1379.
- Millett, P. J., Wilcox III, R. B., O’holleran, J. D., & Warner, J. J. (2006). Rehabilitation of the Rotator Cuff: An Evaluation‐Based Approach. Journal of the American Academy of Orthopaedic Surgeons, 14(11), 599-609.
- Gerber, C., Fuchs, B., & Hodler, J. (2000). The Results of Repair of Massive Tears of the Rotator Cuff*. The Journal of Bone & Joint Surgery, 82(4), 505-505.
- Boileau, P., Brassart, N., Watkinson, D. J., Carles, M., Hatzidakis, A. M., & Krishnan, S. G. (2005). Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal?. J Bone Joint Surg Am, 87(6), 1229-1240.
- Brotzman, SB, Wilk, KE, Mosby. (2003). Clinical Orthopaedic Rehabilitation, 2nd Edition
- Cofield, R. H., Parvizi, J., Hoffmeyer, P. J., Lanzer, W. L., Ilstrup, D. M., & Rowland, C. M. (2001). Surgical repair of chronic rotator cuff tears. The Journal of Bone & Joint Surgery, 83(1), 71-71.
- Ellman, H., Hanker, G., & Bayer, M. (1986). Repair of the rotator cuff. End-result study of factors influencing reconstruction. J Bone Joint Surg Am, 68(8), 1136-1144.
- Hawkins, R. J., Misamore, G. W., & Hobeika, P. E. (1985). Surgery for full-thickness rotator-cuff tears. J Bone Joint Surg Am, 67(9), 1349-1355.
- Neer, C. S., Flatow, E. L., & Lech, O. (1988). Tears of the rotator cuff: long term results of anterior acromioplasty and repair. Orthop Trans, 12(735), 156.
- Hawkins, R. J., Misamore, G. W., & Hobeika, P. E. (1985). Surgery for full-thickness rotator-cuff tears. J Bone Joint Surg Am, 67(9), 1349-1355.
- Cofield, R. H., Parvizi, J., Hoffmeyer, P. J., Lanzer, W. L., Ilstrup, D. M., & Rowland, C. M. (2001). Surgical repair of chronic rotator cuff tears. The Journal of Bone & Joint Surgery, 83(1), 71-71.
- Gartsman, G. M. (1998). Arthroscopic management of rotator cuff disease. Journal of the American Academy of Orthopaedic Surgeons, 6(4), 259-266.
- Gartsman, G. M., Brinker, M. R., & Khan, M. (1998). Early effectiveness of arthroscopic repair for full-thickness tears of the rotator cuff. An outcome analysis. J Bone Joint Surg Am, 80(1), 33-40.
- Gartsman, G. M., & Hammerman, S. M. (1997). Full-thickness tears: arthroscopic repair. Orthopedic Clinics of North America, 28(1), 83-98.
- Yamaguchi, K., Levine, W. N., Marra, G., Galatz, L. M., Klepps, S., & Flatow, E. (2003). Transitioning to arthroscopic rotator cuff repair: the pros and cons. J Bone Joint Surg Am, 85(1), 144-155.
- Rokito, A. S., Cuomo, F., Gallagher, M. A., & Zuckerman, J. D. (1999). Long-term functional outcome of repair of large and massive chronic tears of the rotator cuff. The Journal of Bone & Joint Surgery, 81(7), 991-7.
- Rokito, A. S., Zuckerman, J. D., Gallagher, M. A., & Cuomo, F. (1996). Strength after surgical repair of the rotator cuff. Journal of shoulder and elbow surgery, 5(1), 12-17.
- Mohtadi, N. G., Hollinshead, R. M., Sasyniuk, T. M., Fletcher, J. A., Chan, D. S., & Li, F. X. (2008). A randomized clinical trial comparing open to arthroscopic acromioplasty with mini-open rotator cuff repair for full-thickness rotator cuff tears disease-specific quality of life outcome at an average 2-year follow-up. The American journal of sports medicine, 36(6), 1043-1051.
- Watson, M. (1985). Major ruptures of the rotator cuff. The results of surgical repair in 89 patients. Bone & Joint Journal, 67(4), 618-624.
- Fealy, S., Kingham, T. P., & Altchek, D. W. (2002). Mini-open rotator cuff repair using a two-row fixation technique: outcomes analysis in patients with small, moderate, and large rotator cuff tears. Arthroscopy: The Journal of Arthroscopic & Related Surgery, 18(6), 665-670.
- Ellenbecker, T. S., Elmore, E., & Bailie, D. S. (2006). Descriptive report of shoulder range of motion and rotational strength 6 and 12 weeks following rotator cuff repair using a mini-open deltoid splitting technique. Journal of Orthopaedic & Sports Physical Therapy, 36(5), 326-335.
- Baker, C. L., & Liu, S. H. (1995). Comparison of open and arthroscopically assisted rotator cuff repairs. The American journal of sports medicine, 23(1), 99-104.
- Paulos, L. E., & Kody, M. H. (1994). Arthroscopically enhanced” miniapproach” to rotator cuff repair. The American journal of sports medicine, 22(1), 19-25.
- Posada, A., Uribe, J. W., Hechtman, K. S., Tjin-A-Tsoi, E. W., & Zvijac, J. E. (2000). Mini–Deltoid Splitting Rotator Cuff Repair: Do Results Deteriorate With Time?. Arthroscopy: The Journal of Arthroscopic & Related Surgery, 16(2), 137-141.
- Morse, K., Davis, A. D., Afra, R., Kaye, E. K., Schepsis, A., & Voloshin, I. (2008). Arthroscopic Versus Mini-open Rotator Cuff Repair A Comprehensive Review and Meta-analysis. The American journal of sports medicine, 36(9), 1824-1828.
- Yamaguchi, K., Levine, W. N., Marra, G., Galatz, L. M., Klepps, S., & Flatow, E. (2003). Transitioning to arthroscopic rotator cuff repair: the pros and cons. J Bone Joint Surg Am, 85(1), 144-155.
- Gartsman, G. M., Khan, M., & Hammerman, S. M. (1998). Arthroscopic repair of full-thickness tears of the rotator cuff. J Bone Joint Surg Am, 80(6), 832-40.
- Lindley, K., & Jones, G. L. (2010). Outcomes of arthroscopic versus open rotator cuff repair: a systematic review of the literature. American journal of orthopedics (Belle Mead, NJ), 39(12), 592-600.
- Morse, K., Davis, A. D., Afra, R., Kaye, E. K., Schepsis, A., & Voloshin, I. (2008). Arthroscopic Versus Mini-open Rotator Cuff Repair A Comprehensive Review and Meta-analysis. The American journal of sports medicine, 36(9), 1824-1828.
- Colegate-Stone, T., Allom, R., Tavakkolizadeh, A., & Sinha, J. (2009). An analysis of outcome of arthroscopic versus mini-open rotator cuff repair using subjective and objective scoring tools. Knee Surgery, Sports Traumatology, Arthroscopy, 17(6), 691-694.
- Boileau, P., Brassart, N., Watkinson, D. J., Carles, M., Hatzidakis, A. M., & Krishnan, S. G. (2005). Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal?. J Bone Joint Surg Am, 87(6), 1229-1240.
- Oh, L. S., Wolf, B. R., Hall, M. P., Levy, B. A., & Marx, R. G. (2007). Indications for rotator cuff repair: a systematic review. Clinical orthopaedics and related research, 455, 52-63.
- Clemnent N, McBirnie J. Focus on Rotator Cuff Tears. British Editorial Society of Bone and Joint Surgery. 2012.
- Mansat P, Cofield RH, Kersten TE, Rowland CM. Complications of rotator cuff repair. Orthop Clin North Am 1997;28:205-13.
- Bellumore, Y., Mansat, M., & Assoun, J. (1993). Results of the surgical repair of the rotator cuff. Radio-clinical correlation. Revue de chirurgie orthopedique et reparatrice de l’appareil moteur, 80(7), 582-594.
- Harryman, D. 2., Mack, L. A., Wang, K. Y., Jackins, S. E., Richardson, M. L., & Matsen, F. 3. (1991). Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. The Journal of Bone & Joint Surgery, 73(7), 982-989.
- Galatz, L. M., Ball, C. M., Teefey, S. A., Middleton, W. D., & Yamaguchi, K. (2004). The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am, 86(2), 219-224.
- Gerber, C., Fuchs, B., & Hodler, J. (2000). The Results of Repair of Massive Tears of the Rotator Cuff*. The Journal of Bone & Joint Surgery, 82(4), 505-505.
- Thomazeau, H., Boukobza, E., Morcet, N., Chaperon, J., & Langlais, F. (1997). Prediction of rotator cuff repair results by magnetic resonance imaging. Clinical orthopaedics and related research, 344, 275-283.
- Harryman, D. 2., Mack, L. A., Wang, K. Y., Jackins, S. E., Richardson, M. L., & Matsen, F. 3. (1991). Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. The Journal of Bone & Joint Surgery, 73(7), 982-989.
- Fealy, S., Kingham, T. P., & Altchek, D. W. (2002). Mini-open rotator cuff repair using a two-row fixation technique: outcomes analysis in patients with small, moderate, and large rotator cuff tears. Arthroscopy: The Journal of Arthroscopic & Related Surgery, 18(6), 665-670.
- Djurasovic, M., Marra, G., Arroyo, J. S., Pollock, R. G., Flatow, E. L., & Bigliani, L. U. (2001). Revision rotator cuff repair: factors influencing results. J Bone Joint Surg Am, 83(12), 1849-1855.
- Jost, B., Zumstein, M., Pfirrmann, C. W., & Gerber, C. (2006). Long-term outcome after structural failure of rotator cuff repairs. J Bone Joint Surg Am, 88(3), 472-479.
- Kim, D. H., ElAttrache, N. S., Tibone, J. E., Jun, B. J., DeLaMora, S. N., Kvitne, R. S., & Lee, T. Q. (2006). Biomechanical comparison of a single-row versus double-row suture anchor technique for rotator cuff repair. The American journal of sports medicine, 34(3), 407-414.
- Mazzocca, A. D., Millett, P. J., Guanche, C. A., Santangelo, S. A., & Arciero, R. A. (2005). Arthroscopic single-row versus double-row suture anchor rotator cuff repair. The American journal of sports medicine, 33(12), 1861-1868.
- Cole, B. J., ElAttrache, N. S., & Anbari, A. (2007). Arthroscopic rotator cuff repairs: an anatomic and biomechanical rationale for different suture-anchor repair configurations. Arthroscopy: The Journal of Arthroscopic & Related Surgery, 23(6), 662-669.
- Kim, D. H., ElAttrache, N. S., Tibone, J. E., Jun, B. J., DeLaMora, S. N., Kvitne, R. S., & Lee, T. Q. (2006). Biomechanical comparison of a single-row versus double-row suture anchor technique for rotator cuff repair. The American journal of sports medicine, 34(3), 407-414.
- Milano, G., Grasso, A., Zarelli, D., Deriu, L., Cillo, M., & Fabbriciani, C. (2008). Comparison between single-row and double-row rotator cuff repair: a biomechanical study. Knee Surgery, Sports Traumatology, Arthroscopy, 16(1), 75-80.
- Nelson, C. O., Sileo, M. J., Grossman, M. G., & Serra-Hsu, F. (2008). Single-row modified mason-allen versus double-row arthroscopic rotator cuff repair: a biomechanical and surface area comparison. Arthroscopy: The Journal of Arthroscopic & Related Surgery, 24(8), 941-948.
- Trappey, G. J., & Gartsman, G. M. (2011). A systematic review of the clinical outcomes of single row versus double row rotator cuff repairs. Journal of shoulder and elbow surgery, 20(2), S14-S19.
- Franceschi, F., Ruzzini, L., Longo, U. G., Martina, F. M., Beomonte Zobel, B., Maffulli, N., & Denaro, V. (2007). Equivalent clinical results of arthroscopic single-row and double-row suture anchor repair for rotator cuff tears. The American journal of sports medicine, 35(8), 1254-1260.
- Gartsman, G. M., Drake, G., Edwards, T. B., Elkousy, H. A., Hammerman, S. M., O’Connor, D. P., & Press, C. M. (2013). Ultrasound evaluation of arthroscopic full-thickness supraspinatus rotator cuff repair: single-row versus double-row suture bridge (transosseous equivalent) fixation. Results of a prospective, randomized study. Journal of Shoulder and Elbow Surgery, 22(11), 1480-1487.
- Park, J. Y., Lhee, S. H., Choi, J. H., Park, H. K., Yu, J. W., & Seo, J. B. (2008). Comparison of the clinical outcomes of single-and double-row repairs in rotator cuff tears. The American journal of sports medicine, 36(7), 1310-1316.
- Rodeo, S. A., Arnoczky, S. P., Torzilli, P. A., Hidaka, C., & Warren, R. F. (1993). Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. JBJS, 75(12), 1795-1803.
- Hatakeyama, Y., Itoi, E., Pradhan, R. L., Urayama, M., & Sato, K. (2001). Effect of Arm Elevation and Rotation on the Strain in the Repaired Rotator Cuff Tendon A Cadaveric Study. The American journal of sports medicine, 29(6), 788-794.
- Rathbun, J. B., & Macnab, I. (1970). The microvascular pattern of the rotator cuff. Bone & Joint Journal, 52(3), 540-553.
- Speer, K. P., Warren, R. F., & Horowitz, L. (1996). The efficacy of cryotherapy in the postoperative shoulder. Journal of Shoulder and Elbow Surgery, 5(1), 62-68.
- Heiderscheit, B. C., Sherry, M. A., Silder, A., Chumanov, E. S., & Thelen, D. G. (2010). Hamstring strain injuries: recommendations for diagnosis, rehabilitation, and injury prevention. journal of orthopaedic & sports physical therapy, 40(2), 67-81.
- Magnusson, S. P., Hansen, P., & Kjaer, M. (2003). Tendon properties in relation to muscular activity and physical training. Scandinavian journal of medicine & science in sports, 13(4), 211-223.
- Kelly, B. T., Roskin, L. A., Kirkendall, D. T., & Speer, K. P. (2000). Shoulder muscle activation during aquatic and dry land exercises in nonimpaired subjects. Journal of Orthopaedic & Sports Physical Therapy, 30(4), 204-210.
- Brady, B., Redfern, J., Macdougal, G., & Williams, J. (2008). The addition of aquatic therapy to rehabilitation following surgical rotator cuff repair: a feasibility study. Physiotherapy Research International, 13(3), 153-161.
- Brotzman, SB, Wilk, KE, Mosby. (2003). Clinical Orthopaedic Rehabilitation, 2nd Edition
- Millett, P. J., Wilcox III, R. B., O’holleran, J. D., & Warner, J. J. (2006). Rehabilitation of the Rotator Cuff: An Evaluation‐Based Approach. Journal of the American Academy of Orthopaedic Surgeons, 14(11), 599-609.
- Carpenter, J. E., Thomopoulos, S., Flanagan, C. L., DeBano, C. M., & Soslowsky, L. J. (1998). Rotator cuff defect healing: a biomechanical and histologic analysis in an animal model. Journal of Shoulder and Elbow Surgery, 7(6), 599-605.
- Lewis, C. W., Schlegel, T. F., Hawkins, R. J., James, S. P., & Turner, A. S. (2000). The effect of immobilization on rotator cuff healing using modified Mason-Allen stitches: a biomechanical study in sheep. Biomedical sciences instrumentation, 37, 263-268.
- Reinold, M. M., Macrina, L. C., Wilk, K. E., Dugas, J. R., Cain, E. L., & Andrews, J. R. (2008). The effect of neuromuscular electrical stimulation of the infraspinatus on shoulder external rotation force production after rotator cuff repair surgery. The American journal of sports medicine, 36(12), 2317-2321.
- Laudner, K., Compton, B. D., McLoda, T. A., & Walters, C. M. (2014). Acute effects of instrument assisted soft tissue mobilization for improving posterior shoulder range of motion in collegiate baseball players. International journal of sports physical therapy, 9(1), 1.
- Brotzman, SB, Wilk, KE, Mosby. (2003). Clinical Orthopaedic Rehabilitation, 2nd Edition