At VASTA, we are naturally skeptical of ‘new’ treatment approaches, especially those that make BIG promises. Our clinicians are current with best practices and are committed to an evidence-based approach.
At the same time, we work hard to stay on the cutting edge of emerging treatment techniques.
Blood Flow Restriction (BFR) Training is one such approach.
Recently, this little known technique has become a popular tool at military hospitals for soldiers who experienced significant limb trauma (e.g. in ‘limb salvage’ wards) and with amputees. Millions of dollars of research has been poured into exploring the utility of BFR for patients with significant limitations (i.e. limited mobility and/or limited ability to bear weight) who need to build muscle. A very difficult task!
Similarly, our patients with post-operative limitation that prohibits weight bearing or heavy use of the LE for weeks (or even months) end up with dramatic muscle atrophy. Previously thought of as a ‘necessary evil’ to protect the surgical repair… Now, maybe not!
BFR involves restricting the blood flow to a limb during exercise in order to starve the muscle of oxygen, thus causing a number of ‘pro-growth’ chemical and hormonal changes. This allows us to stimulate muscle hypertrophy (growth) with very low intensity & low load exercises.
For any patient requiring a prolonged period of ‘rest’, immobilization or limited weight bearing – we believe BFR training has the potential to be a game changer.
What is Blood Flow Restriction Training?
Blood flow to the limb is significantly reduced, and blood return (flow out of the limb) is completely stopped, for a brief period of time, with the use of a tourniquet. This specially designed tourniquet contains a Doppler (to sense blood flow) and is automated to control blood flow to precisely defined parameters.
Once blood flow is reduced to the limb, the patient performs ~ 4-5 minutes of low intensity resistance training (LIT) exercises. While the load is very light – and thus the strain to the bone, cartilage, ligaments, etc. is minimal – the muscle gets exhausted! This is because it is being forced to work under anaerobic conditioning (without oxygen from fresh blood supply). Thus, the muscle seems to work very hard, all muscle fiber types are recruited, lactic acid builds up, the muscle glycogen levels become depleted and the exerciser feels as though they have just worked out really hard.
More importantly, the muscle also thinks it worked out really hard; and, the response is similar to high intensity training (HIT) – The muscle grows and gets stronger!
Bottom line – this technology allows us to achieve strength and hypertrophy (size) gains that, under normal circumstances, are possible only under high loads & high exercise intensities.
We can make gains earlier and faster, even when an injury prevents you from exercising.
For those looking for more detail and a review of the extensive research that has been done in this field, read on…
Graph 1
Graph 2
According to the American College of Sports Medicine (ACSM), building muscle strength and size can be achieved through moderate to high intensities (Donnelly 2009) typically at intensities of 65% > 70% of a ‘1 rep max’.
Unfortunately, many of our injured athletes and post-operative patients can not work at that level of intensity. Worst, patients that require prolonged immobilization and/or restricted weight bearing on a limb suffer severe muscle atrophy.
Low intensity exercise, which is tolerated much better in these folks, does not result in strength gains or muscle hypertrophy.
However, exercising at low intensities (LIT) has been shown to result in strength gains and hypertrophy when coupled with BFR!
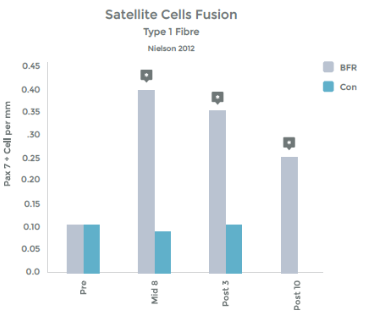
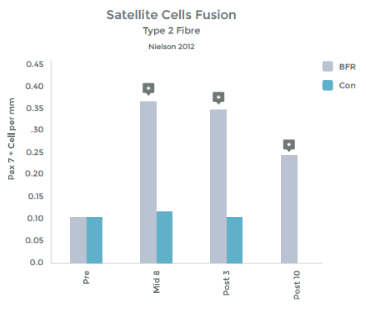
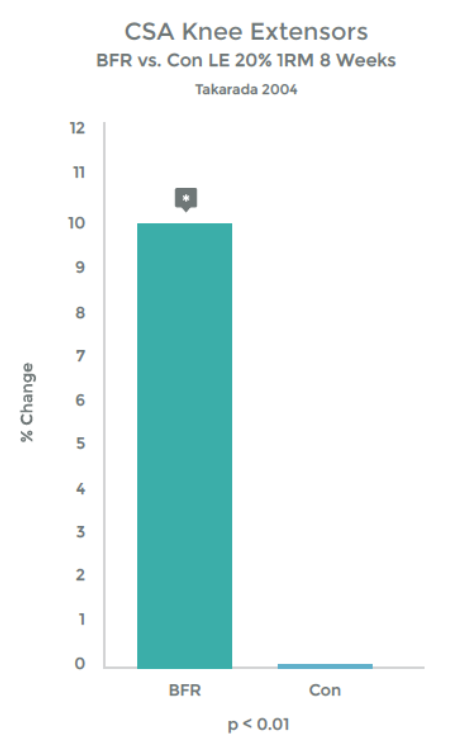
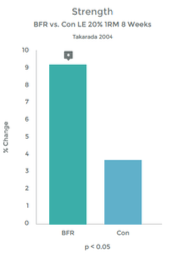
Graph 1 & 2 to left illustrate this finding.
Strength gains & Hypertrophy in the Quadriceps muscle from VERY low load (20% 1RM) exercise over 8 weeks!
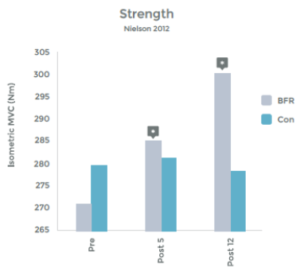
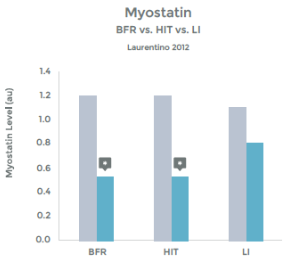
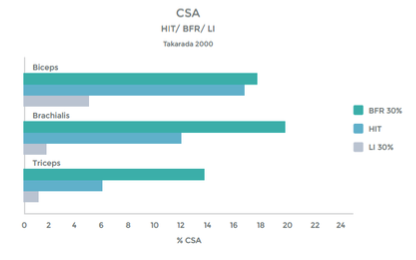
Research comparing the effects of LIT with BFR to High Intensity Training (HIT) helps illustrate the effectiveness even better. This research was done on arm muscles, and the researchers included LIT without BFR as a comparison.1 . See graphs 3 & 4 to the right.
As you can see in the graphs to the right, the changes in strength and hypertrophy of a muscle subjected to BFR training are very similar to the effects seen with High Intensity loading. In fact, the BFR group showed even greater initial changes in muscle ‘cross sectional area’ – Growth!
Many studies have demonstrated significant increases in muscle strength using BFR and exercise, as compared to exercise alone, at low intensity. 2 3 4 5 6 7 8 9 10
Graph 3
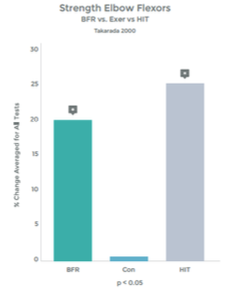
Graph 4
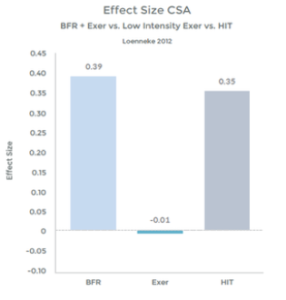
Graph 5
Many studies have demonstrated significant increases in muscle hypertrophy using BFR and exercise, as compared to exercise alone, at low intensity 11 12 13 14 15 16 17 18
Many studies have demonstrated that the effects of BFR with LIT are similar to HIT with respect to strength and hypertrophy gains. 19 20 21 22 23 24 25 26
How Does it Work?
From the available research – which is vast – we are left to believe that multiple mechanisms may be at work. Below is a summary:
1) Lactate Production
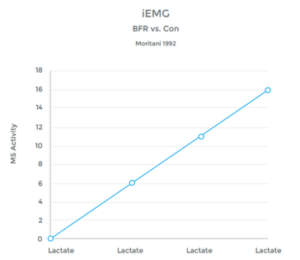
The accumulation of lactic acid in a muscle is a result of ‘anaerobic’ metabolism – work being performed without adequate oxygen. This provides the ‘burn’ of a hard workout to fatigue.
When O2 is plentiful, the muscle utilizes the Kreb Cycle to produce energy needed to perform work. However, when O2 is depleted, the muscle utilizes an alternative mechanism called the Cori Cycle (also known as the Lactic Acid Cycle). Lactate is a by-product of this process.
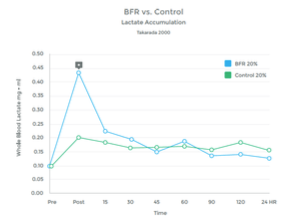
Graph 6
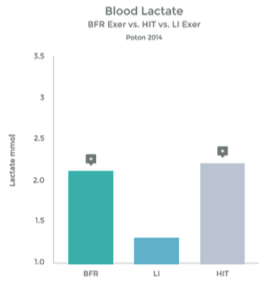
Graph 7
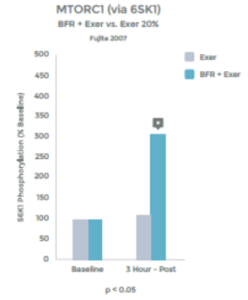
While the Cori Cycle is typically only activated under high intensity conditions, BFR training appears to be an alternative way to achieve a similar rise in lactate levels. See graphs 6 & 7 to the right.
Many studies have observed this rise in lactic acid associated with BFR Training. 27 28 29 30 31 32 33
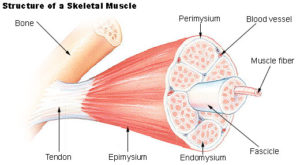
Image 1
Graph 8
2) Improved Muscle Activation
Typically, under low loads, a relatively small amount of the muscle is ‘recruited’ to perform the work. Muscles are made up of hundreds of thousands of individual muscle fibers, which are, themselves, sub-categorized into muscle ‘fascicles’. (See image to left)
Nerves that innervate the muscle do so by branching out and forming many attachment points. These separate connections are known as ‘motor units’ and they are capable of contracting separate from the other portions of the muscle. A given muscle will have hundreds of motor units. So, with light contraction, a small number of ‘motor units’ are recruited (or turned on), but with heavy effort, more units are enlisted.
Under typical conditions, lactic acid builds up in the muscle only under heavy, fatiguing conditions, thus the body responds to the presence of lactate by up regulating (increasing) motor unit recruitment. The body recruits, not just higher numbers of motor units, but large motor units as well (the ‘units’ with lots of fibers in them, that typically get called to action under high loads)
Studies have shown a clear link between the presence of lactic acid, and an increase in muscle fiber recruitment. (See graph 8 on left)
Anyone who has had knee surgery and couldn’t get the Quad to “wake up” can tell you – this is a big deal!
Multiple studies have confirmed the direct correlation between BFR training and a strong Lactic Acid response. If you doubt it… come try it! You will be sold.
3) Increased activation of ‘Fast Twitch’ Fibers
Under normal conditions, LIT does not recruit these muscle fiber types. ‘Fast Twitch’ muscles are the fibers that fire hard and heavy when a lot of work is needed quickly. They do not rely on oxygen for fuel, as apposed to ‘Slow Twitch’ endurance fibers. With low loads, these motor units will be recruited only when starved of Oxygen with BFR. 34
4) Increased Release of Growth Hormone
Growth Hormone (GH) appears to play an important role in muscle recovery. Despite the commonly held belief that GH builds muscle, what it appears to be primarily involved in is the regeneration of Muscle Collagen and Tendon health 35 36 37 38
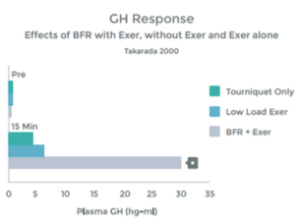
An accumulation of Lactate – as occurs with HIT and with LIT with BFR – triggers augmented growth hormone release. 39 40 41 42. When the muscle is in use, it stimulates nerve endings within the muscle (known as Type III and Type IV afferent nerves) that communicate with many areas in the body (e.g. your heart and lungs). We know that with heavy use, and the resulting Lactic Acid build up, these nerve stimulate the Pituitary Gland to release Growth Hormone 43. Low Intensity Training with BFR appears to result in a similar response. Please see graph 9.
The results of the study above were replicated by a second study that found, with BFR + LIT, GH levels 290% higher than baseline (resting measurements). This response is 1.7 x than that previously reported to be associated with HIT 44 45
Graph 9
Graph 10
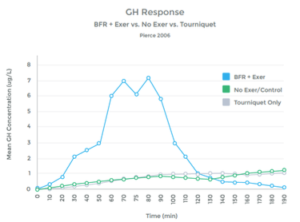
Multiple studies have shown a similar response, as well as a lack of hormonal response when LIT was not combined with BFR. (See graph 10 to left). 46 47 48 49 50 51 52 53 54 55
Thus with BFR training, the body interprets the Lactate build up as an indicator of heavy use and muscle breakdown. The response is to increase the release of GH as a mechanism to repair and regenerate – however, there was significant tissue damage associated with LIT with BFR, thus, the patient ends up with a positive collagen turnover (This is good!)
5) Increased Release of Numerous Hormonal Triggers for Muscle Growth
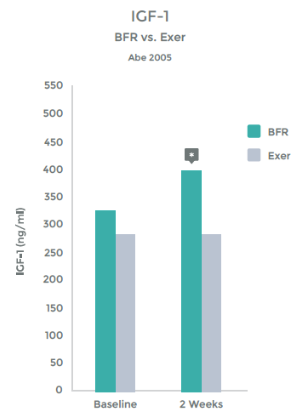
Another hormonal benefit from BFR is that the increase in GH stimulates the release of Insulin like growth factor (IGF-1). IGF-1 does effect muscle hypertrophy (growth), strength gains and some researchers have dubbed it the regulator of muscle mass 56 57 58 59
Multiple studies have shown an increase of IGF-1 levels when BFR is added to exercise.60 61 62
Studies have even shown an increase in IGF-1 levels with LIT + BFR greater than that seen with HIT 63 64 65
Abe, et al demonstrated this – See graph 11 on right.
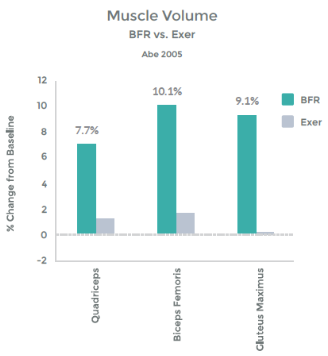
In addition, they illustrated the link b/w this hormonal response and muscle hypertrophy. This graph demonstrates the percent change in Cross Sectional Area (CSA), i.e. muscle size with LIT Vs. LIT + BFR. – See graph 12 on right.
So, with BFR training, we trigger the release of GH. This, in turn, activates IGF-1 and muscle stem cells to facilitate muscle hypertrophy 66 – resulting in a bigger, stronger muscle!
Graph 11
Graph 12